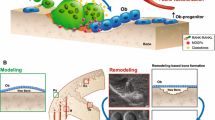
Abstract
Cathepsin K is the protease that is primarily responsible for the degradation of bone matrix by osteoclasts. Inhibitors of cathepsin K are in development for treatment of osteoporosis. Currently available antiresorptive drugs interfere with osteoclast function. They inhibit both bone resorption and formation, due to the coupling between these processes. Cathepsin K inhibitors, conversely, target the resorption process itself and may not interfere with osteoclast stimulation of bone formation. In fact, when cathepsin K is absent or inhibited in mice, rabbits, or monkeys, bone formation is maintained or increased. In humans, inhibition of cathepsin K is associated with sustained reductions in bone resorption markers but with smaller and transient reductions in bone formation markers. The usefulness of cathepsin K inhibitors in osteoporosis is now being examined in phase 2 and phase 3 clinical trials of postmenopausal osteoporotic women.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Rachner TD, Khosla S, Hofbauer LC. Osteoporosis: now and the future. Lancet. 2011;377:1276–87.
Sambrook P, Cooper C. Osteoporosis. Lancet. 2006;367:2010–8.
Cummings SR, Melton LJ. Epidemiology and outcomes of osteoporotic fractures. Lancet. 2002;359:1761–7.
Fast Facts. nof.org/node/40. Accessed 7 June 2011.
Teitelbaum SL. Bone resorption by osteoclasts. Science. 2000;289:1504–8.
Vaananen HK, Laitala-Leinonen T. Osteoclast lineage and function. Arch Biochem Biophys. 2008;473:132–8.
Lecaille F, Bromme D, Lalmanach G. Biochemical properties and regulation of cathepsin K activity. Biochimie. 2008;90:208–26.
Karsdal MA, Martin TJ, Bollerslev J, et al. Are nonresorbing osteoclasts sources of bone anabolic activity? J Bone Miner Res. 2007;22:487–94.
Henriksen K, Neutzsky-Wulff AV, Bonewald LF, Karsdal MA. Local communication on and within bone controls bone remodeling. Bone. 2009;44:1026–33.
Matsuo K, Irie N. Osteoclast-osteoblast communication. Arch Biochem Biophys. 2008;473:201–9.
Boyce BF, Xing L. Functions of RANKL/RANK/OPG in bone modeling and remodeling. Arch Biochem Biophys. 2008;473:139–46.
Fuller K, Lawrence KM, Ross JL, et al. Cathepsin K inhibitors prevent matrix-derived growth factor degradation by human osteoclasts. Bone. 2008;42:200–11.
Tang Y, Wu X, Lei W, et al. TGF-beta1-induced migration of bone mesenchymal stem cells couples bone resorption with formation. Nat Med. 2009;15:757–65.
Pasquale EB. Eph-ephrin bidirectional signaling in physiology and disease. Cell. 2008;133:38–52.
Zhao C, Irie N, Takada Y, et al. Bidirectional ephrinB2-EphB4 signaling controls bone homeostasis. Cell Metab. 2006;4:111–21.
Segovia-Silvestre T, Neutzsky-Wulff AV, Sorensen MG, et al. Advances in osteoclast biology resulting from the study of osteopetrotic mutations. Hum Genet. 2009;124:561–77.
Drake FH, Dodds RA, James IE, et al. Cathepsin K, but not cathepsins B, L, or S, is abundantly expressed in human osteoclasts. J Biol Chem. 1996;271:12511–6.
Bromme D, Okamoto K, Wang BB, Biroc S. Human cathepsin O2, a matrix protein-degrading cysteine protease expressed in osteoclasts. Functional expression of human cathepsin O2 in Spodoptera frugiperda and characterization of the enzyme. J Biol Chem. 1996;271:2126–32.
Garnero P, Borel O, Byrjalsen I, et al. The collagenolytic activity of cathepsin K is unique among mammalian proteinases. J Biol Chem. 1998;273:32347–52.
Gelb BD, Shi GP, Chapman HA, Desnick RJ. Pycnodysostosis, a lysosomal disease caused by cathepsin K deficiency. Science. 1996;273:1236–8.
Schilling AF, Mulhausen C, Lehmann W, et al. High bone mineral density in pycnodysostotic patients with a novel mutation in the propeptide of cathepsin K. Osteoporos Int. 2007;18:659–69.
Ho N, Punturieri A, Wilkin D, et al. Mutations of CTSK result in pycnodysostosis via a reduction in cathepsin K protein. J Bone Miner Res. 1999;14:1649–53.
Saftig P, Hunziker E, Wehmeyer O, et al. Impaired osteoclastic bone resorption leads to osteopetrosis in cathepsin-K-deficient mice. Proc Natl Acad Sci U S A. 1998;95:13453–8.
Gowen M, Lazner F, Dodds R, et al. Cathepsin K knockout mice develop osteopetrosis due to a deficit in matrix degradation but not demineralization. J Bone Miner Res. 1999;14:1654–63.
Li CY, Jepsen KJ, Majeska RJ, et al. Mice lacking cathepsin K maintain bone remodeling but develop bone fragility despite high bone mass. J Bone Miner Res. 2006;21:865–75.
Pennypacker B, Shea M, Liu Q, et al. Bone density, strength, and formation in adult cathepsin K (-/-) mice. Bone. 2009;44:199–207.
Kiviranta R, Morko J, Uusitalo H, et al. Accelerated turnover of metaphyseal trabecular bone in mice overexpressing cathepsin K. J Bone Miner Res. 2001;16:1444–52.
Yasuda Y, Kaleta J, Bromme D. The role of cathepsins in osteoporosis and arthritis: rationale for the design of new therapeutics. Adv Drug Deliv Rev. 2005;57:973–93.
Stoch SA, Wagner JA. Cathepsin K inhibitors: a novel target for osteoporosis therapy. Clin Pharmacol Ther. 2008;83:172–6.
Yamashita DS, Marquis RW, Xie R, et al. Structure activity relationships of 5-, 6-, and 7-methyl-substituted azepan-3-one cathepsin K inhibitors. J Med Chem. 2006;49:1597–612.
Gauthier JY, Chauret N, Cromlish W, et al. The discovery of odanacatib (MK-0822), a selective inhibitor of cathepsin K. Bioorg Med Chem Lett. 2008;18:923–8.
Adami S, Supronik J, Hala T, et al. Effect of 1 year treatment with the Cathepsin-K inhibitor, balicatib, on bone mineral density (BMD) in postmenopausal women with osteopenia/osteoporosis. J Bone Miner Res. 2006;21(Suppl S1):S24. Abstract 1085.
Peroni A, Zini A, Braga V, et al. Drug-induced morphea: report of a case induced by balicatib and review of the literature. J Am Acad Dermatol. 2008;59:125–9.
Runger TM, Adami S, Benhamou CL, et al. Morphea-like skin reactions in patients treated with the cathepsin K inhibitor balicatib. J Am Acad Dermatol. 2011. doi:10.1016/j.jaad2010.11.033
Pennypacker BL, Duong lT, Cusick TE, et al. Cathepsin K inhibitors prevent bone loss in estrogen-deficient rabbits. J Bone Miner Res. 2011;26:252–62.
Stroup GB, Kumar S, Jerome CP. Treatment with a potent cathepsin K inhibitor preserves cortical and trabecular bone mass in ovariectomized monkeys. Calcif Tissue Int. 2009;85:344–55.
• Masarachia PJ, Pennypacker B, Pickarski M, et al. Odanacatib reduces bone turnover and increases bone mass in lumbar spine of skeletally mature ovariectomized rhesus monkeys. J Bone Miner Res. 2011, in press. Uses non-human primates to explore the mechanism of action of cathepsin K inhibitors for osteoporosis.
Yamada H, Mori H, Kunishige A, et al. Efficacy of ONO-5334, a cathepsin K inhibitor, on bone turnover markers and bone mineral density in ovariectomized cynomolgus monkeys. Presented at European Calicified Tissue International, Glasgow, Scottland; 2010.
Yamada H, Ochi Y, Kunishige A, et al. Efficacy of ONO-5334, a cathepsin K inhibitor, on bone mass and strength in ovariectomized cynomolgus monkeys. Bone. 2011;48:S221.
Ochi Y, Yamada H, Kunishige ANS, et al. Efficacy of ONO-5334, a cathepsin K inhibitor, on bone turnover and cortical geometry in ovariectomized cynolmolgus monkeys. Bone. 2011;48:S72.
Mayhew PM, Thomas CD, Clement JG, et al. Relation between age, femoral neck cortical stability, and hip fracture risk. Lancet. 2005;366:129–35.
• Jerome C, Missbach M, Gamse R. Balicatib, a cathepsin K inhibitor, stimulates periosteal bone formation in monkeys. Osteoporos Int. 2011.
• Cusick T, Chen CM, Pennypacker BL, et al. Odanacatib treatment increases Hi bone mass and cortical thickness by preserving endocortical bone formation and stimulating periosteal bone formation in ovariectomized adult rhesus monkey. J Bone Miner Res. 2011, in press. Uses non-human primates to explore the mechanism of action of cathepsin K inhibitors for osteoporosis.
•• Bone HG, McClung MR, Roux C, et al. Odanacatib, a cathepsin-K inhibitor for osteoporosis: a two-year study in postmenopausal women with low bone density. J Bone Miner Res. 2010;25:937–47. Reports clinical results of drugs in development that use the novel mechanism of action of inhibition of cathepsin K.
•• Eisman JA, Bone HG, Hosking DJ, et al. Odanacatib in the treatment of postmenopausal women with low bone mineral density: three-year continued therapy and resolution of effect. J Bone Miner Res. 2011;26:242–51. Reports clinical results of drugs in development that use the novel mechanism of action of inhibition of cathepsin K.
Binkley N, Bone H, Gilcrist N, et al. Treatment with the cathepsin K inhibitor odanacatib in postmenopausal women with low BMD: 5 year results of a phase 2 trial. Presented at the American Society of Bone and Mineral Research, San Diego, CA; 2011.
Bone H, Dempster D, Eisman J, et al. Phase 3 fracture trial of odanacatib for osteoporosis - study design. Presented at the Endocrine Society meeting, San Diego, CA; 2010.
•• Eastell R, Nagase S, Ohyama M, et al. Safety and efficacy of the Cathepsin K inhibitor, ONO-5334, in postmenopausal osteoporosis - the OCEAN study. J Bone Miner Res. 2011;26:1303–12. Reports clinical results of drugs in development that use the novel mechanism of action of inhibition of cathepsin K.
Eastell R, Nagase S, Small M, et al. Effect of the cathepsin K inhibitor ONO-5334 on biochemical markers of bone turnover in the treatment of postmennopausal osteopenia or osteoporosis: 2-year results from the OCEAN atudy. J Bone Miner Res. 2011.
Harris ST, Watts NB, Genant HK, et al. Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: a randomized controlled trial. Vertebral Efficacy With Risedronate Therapy (VERT) Study Group. JAMA. 1999;282:1344–52.
Black DM, Schwartz AV, Ensrud KE, et al. Effects of continuing or stopping alendronate after 5 years of treatment: the Fracture Intervention Trial Long-term Extension (FLEX): a randomized trial. JAMA. 2006;296:2927–38.
Gallagher JC, Rapuri PB, Haynatzki G, Detter JR. Effect of discontinuation of estrogen, calcitriol, and the combination of both on bone density and bone markers. J Clin Endocrinol Metab. 2002;87:4914–23.
Miller PD, Bolognese MA, Lewiecki EM, et al. Effect of denosumab on bone density and turnover in postmenopausal women with low bone mass after long-term continued, discontinued, and restarting of therapy: a randomized blinded phase 2 clinical trial. Bone. 2008;43:222–9.
Stakkestad JA, Lakatos P, Lorenc R, et al. Monthly oral ibandronate is effective and well tolerated after 3 years: the MOBILE long-term extension. Clin Rheumatol. 2008;27:955–60.
Disclosure
Conflicts of interest: S. Boonen: received editorial support from Dr. Rosenberg who is an employee of Merck; has received payment for lectures including service on speakers bureaus for Amgen, Novartis, and Servier; and has received travel/accommodations/meeting expenses from Amgen, Novartis, and Servier for Congress participation; E. Rosenberg: is employed by and has stock options in Merck Sharp & Dohme; F. Claessens: none; D. Vanderschueren: none; S. Papapoulos: has been a board member for Merck & Co Advisory Board, Amgen, Novartis; has been a consultant for Merck & Co, Alliance for Better Bone Health, Amgen, Roche, GlaxoSmithKline; and has received honoraria from all above-mentioned entities in relation to participation in AdB and Consultancies; and has received travel/accommodations expenses covered or reimbursed from all above-mentioned entities in relation to participation in AdB.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Boonen, S., Rosenberg, E., Claessens, F. et al. Inhibition of Cathepsin K for Treatment of Osteoporosis. Curr Osteoporos Rep 10, 73–79 (2012). https://doi.org/10.1007/s11914-011-0085-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11914-011-0085-9