Abstract
Purpose of Review
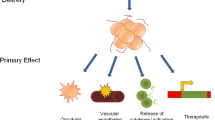
Oncolytic viruses (OVs) exert their antitumor effect through selective killing of cancer cells and induction of host anti-tumor immunity. This review aims to summarize the recent and current trials with OVs for the treatment of lung cancer.
Recent Findings
Several OVs have been developed for the treatment of lung cancer including adenovirus, coxsackievirus B3, reovirus, and vaccinia virus and trials have demonstrated a safe toxicity profile. Early-phase trials in lung cancer with OVs have reported antiviral immune responses and evidence of clinical benefit. However, clinical efficacy of OVs in lung cancer either as monotherapy or in combination with chemotherapy has not been confirmed in larger phase II or III trials. Development of OVs in lung cancer has been limited by difficulty in administering OVs in the tumor directly as well as achieving adequate viral load at all tumor sites with systemically administered OVs.
Summary
Developing novel combinations with OVs, especially checkpoint inhibitors and other immunotherapeutics, may be a strategy to address the limited success seen thus far. Integrating appropriate biomarker studies and meaningful endpoints in future clinical trials will be imperative. Using novel viral delivery systems in addition to increasing tumor specificity through improved genetic modifications in the OVs are other strategies to improve efficacy.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
• Kaufman HL, Kohlhapp FJ, Zloza A. Oncolytic viruses: a new class of immunotherapy drugs. Nat Rev Drug Discov. 2016;15(9):660. https://doi.org/10.1038/nrd.2016.178. This review summarizes the appoach to development of oncolytic viruses for cancer therapy.
Zheng M, Huang J, Tong A, Yang H. Oncolytic viruses for cancer therapy: barriers and recent advances. Mol Ther Oncolytics. 2019;15:234–47. https://doi.org/10.1016/j.omto.2019.10.007.
Prestwich RJ, Errington F, Diaz RM, Pandha HS, Harrington KJ, Melcher AA, Vile RG. The case of oncolytic viruses versus the immune system: waiting on the judgment of Solomon. Hum Gene Ther. 2009;20(10):1119–32. https://doi.org/10.1089/hum.2009.135.
Di Paolo NC, Miao EA, Iwakura Y, Murali-Krishna K, Aderem A, Flavell RA, Papayannopoulou T, Shayakhmetov DM. Virus binding to a plasma membrane receptor triggers interleukin-1 alpha-mediated proinflammatory macrophage response in vivo. Immunity. 2009;31(1):110–21. https://doi.org/10.1016/j.immuni.2009.04.015.
Alberts P, Tilgase A, Rasa A, Bandere K, Venskus D. The advent of oncolytic virotherapy in oncology: the Rigvir(R) story. Eur J Pharmacol. 2018;837:117–26. https://doi.org/10.1016/j.ejphar.2018.08.042.
Liang M. Oncorine, the World First Oncolytic Virus Medicine and its Update in China. Curr Cancer Drug Targets. 2018;18(2):171–6. https://doi.org/10.2174/1568009618666171129221503.
Zeng J, Li X, Sander M, Zhang H, Yan G, Lin Y. Oncolytic viro-immunotherapy: an emerging option in the treatment of gliomas. Front Immunol. 2021;12:721830. https://doi.org/10.3389/fimmu.2021.721830.
• Andtbacka RH, Kaufman HL, Collichio F, Amatruda T, Senzer N, Chesney J, Delman KA, Spitler LE, Puzanov I, Agarwala SS, Milhem M, Cranmer L, Curti B, Lewis K, Ross M, Guthrie T, Linette GP, Daniels GA, Harrington K, Middleton MR, Miller WH Jr, Zager JS, Ye Y, Yao B, Li A, Doleman S, VanderWalde A, Gansert J, Coffin RS. Talimogene laherparepvec improves durable response rate in patients with advanced melanoma. J Clin Oncol. 2015;33(25):2780–8. https://doi.org/10.1200/JCO.2014.58.3377. This trial presented data to support FDA approval of talimogene laherparevec for the treatment of advanced melanoma.
• Ekeke CN, Russell KL, Joubert K, Bartlett DL, Luketich JD, Soloff AC, Guo ZS, Lotze MT, Dhupar R. Fighting fire with fire: oncolytic virotherapy for thoracic malignancies. Ann Surg Oncol. 2021;28(5):2715–27. https://doi.org/10.1245/s10434-020-09477-4. This articles describes the approach to development of oncolytic viruses for the treatment of lung cancer.
Bai Y, Hui P, Du X, Su X. Updates to the antitumor mechanism of oncolytic virus. Thorac Cancer. 2019;10(5):1031–5. https://doi.org/10.1111/1759-7714.13043.
Matsuda T, Karube H, Aruga A. A comparative safety profile assessment of oncolytic virus therapy based on clinical trials. Ther Innov Regul Sci. 2018;52(4):430–7. https://doi.org/10.1177/2168479017738979.
Macedo N, Miller DM, Haq R, Kaufman HL. Clinical landscape of oncolytic virus research in 2020. J Immunother Cancer. 2020;8(2). https://doi.org/10.1136/jitc-2020-001486.
Wang Y, Zhou X, Wu Z, Hu H, Jin J, Hu Y, Dong Y, Zou J, Mao Z, Shi X, Huo Y, Lyu J, Fang Z, Zhang W, Zhu Y, Li B, Liu B. Preclinical safety evaluation of oncolytic herpes simplex virus type 2. Hum Gene Ther. 2019;30(5):651–60. https://doi.org/10.1089/hum.2018.170.
Kimball KJ, Preuss MA, Barnes MN, Wang M, Siegal GP, Wan W, Kuo H, Saddekni S, Stockard CR, Grizzle WE, Harris RD, Aurigemma R, Curiel DT, Alvarez RD. A phase I study of a tropism-modified conditionally replicative adenovirus for recurrent malignant gynecologic diseases. Clin Cancer Res. 2010;16(21):5277–87. https://doi.org/10.1158/1078-0432.CCR-10-0791.
Wei N, Fan JK, Gu JF, He LF, Tang WH, Cao X, Liu XY. A double-regulated oncolytic adenovirus with improved safety for adenocarcinoma therapy. Biochem Biophys Res Commun. 2009;388(2):234–9. https://doi.org/10.1016/j.bbrc.2009.07.142.
Kaufman HL, Kohlhapp FJ, Zloza A. Oncolytic viruses: a new class of immunotherapy drugs. Nat Rev Drug Discov. 2015;14(9):642–62. https://doi.org/10.1038/nrd4663.
Fukuhara H, Ino Y, Todo T. Oncolytic virus therapy: a new era of cancer treatment at dawn. Cancer Sci. 2016;107(10):1373–9. https://doi.org/10.1111/cas.13027.
Guo ZS, Liu Z, Bartlett DL. Oncolytic immunotherapy: dying the right way is a key to eliciting potent antitumor immunity. Front Oncol. 2014;4:74. https://doi.org/10.3389/fonc.2014.00074.
Bellucci R, Martin A, Bommarito D, Wang K, Hansen SH, Freeman GJ, Ritz J. Interferon-gamma-induced activation of JAK1 and JAK2 suppresses tumor cell susceptibility to NK cells through upregulation of PD-L1 expression. Oncoimmunology. 2015;4(6):e1008824. https://doi.org/10.1080/2162402X.2015.1008824.
Bridle BW, Stephenson KB, Boudreau JE, Koshy S, Kazdhan N, Pullenayegum E, Brunelliere J, Bramson JL, Lichty BD, Wan Y. Potentiating cancer immunotherapy using an oncolytic virus. Mol Ther. 2010;18(8):1430–9. https://doi.org/10.1038/mt.2010.98.
Kanerva A, Nokisalmi P, Diaconu I, Koski A, Cerullo V, Liikanen I, Tahtinen S, Oksanen M, Heiskanen R, Pesonen S, Joensuu T, Alanko T, Partanen K, Laasonen L, Kairemo K, Pesonen S, Kangasniemi L, Hemminki A. Antiviral and antitumor T-cell immunity in patients treated with GM-CSF-coding oncolytic adenovirus. Clin Cancer Res. 2013;19(10):2734–44. https://doi.org/10.1158/1078-0432.CCR-12-2546.
Woller N, Gurlevik E, Fleischmann-Mundt B, Schumacher A, Knocke S, Kloos AM, Saborowski M, Geffers R, Manns MP, Wirth TC, Kubicka S, Kuhnel F. Viral infection of tumors overcomes resistance to PD-1-immunotherapy by broadening neoantigenome-directed T-cell responses. Mol Ther. 2015;23(10):1630–40. https://doi.org/10.1038/mt.2015.115.
Reale A, Vitiello A, Conciatori V, Parolin C, Calistri A, Palu G. Perspectives on immunotherapy via oncolytic viruses. Infect Agent Cancer. 2019;14:5. https://doi.org/10.1186/s13027-018-0218-1.
Martuza RL, Malick A, Markert JM, Ruffner KL, Coen DM. Experimental therapy of human glioma by means of a genetically engineered virus mutant. Science. 1991;252(5007):854–6. https://doi.org/10.1126/science.1851332.
Pikor LA, Bell JC, Diallo JS. Oncolytic viruses: exploiting cancer’s deal with the devil. Trends Cancer. 2015;1(4):266–77. https://doi.org/10.1016/j.trecan.2015.10.004.
Shi T, Song X, Wang Y, Liu F, Wei J. Combining oncolytic viruses with cancer immunotherapy: establishing a new generation of cancer treatment. Front Immunol. 2020;11:683. https://doi.org/10.3389/fimmu.2020.00683.
Wang LC, Lynn RC, Cheng G, Alexander E, Kapoor V, Moon EK, Sun J, Fridlender ZG, Isaacs SN, Thorne SH, Albelda SM. Treating tumors with a vaccinia virus expressing IFNbeta illustrates the complex relationships between oncolytic ability and immunogenicity. Mol Ther. 2012;20(4):736–48. https://doi.org/10.1038/mt.2011.228.
Willmon CL, Saloura V, Fridlender ZG, Wongthida P, Diaz RM, Thompson J, Kottke T, Federspiel M, Barber G, Albelda SM, Vile RG. Expression of IFN-beta enhances both efficacy and safety of oncolytic vesicular stomatitis virus for therapy of mesothelioma. Cancer Res. 2009;69(19):7713–20. https://doi.org/10.1158/0008-5472.CAN-09-1013.
Sterman DH, Recio A, Carroll RG, Gillespie CT, Haas A, Vachani A, Kapoor V, Sun J, Hodinka R, Brown JL, Corbley MJ, Parr M, Ho M, Pastan I, Machuzak M, Benedict W, Zhang XQ, Lord EM, Litzky LA, Heitjan DF, June CH, Kaiser LR, Vonderheide RH, Albelda SM, Kanther M. A phase I clinical trial of single-dose intrapleural IFN-beta gene transfer for malignant pleural mesothelioma and metastatic pleural effusions: high rate of antitumor immune responses. Clin Cancer Res. 2007;13(15 Pt 1):4456–66. https://doi.org/10.1158/1078-0432.CCR-07-0403.
Corke L, Sacher A. New strategies and combinations to improve outcomes in immunotherapy in metastatic non-small-cell lung cancer. Curr Oncol. 2021;29(1):38–55. https://doi.org/10.3390/curroncol29010004.
Zhang H, Xie W, Zhang Y, Dong X, Liu C, Yi J, Zhang S, Wen C, Zheng L, Wang H. Oncolytic adenoviruses synergistically enhance anti-PD-L1 and anti-CTLA-4 immunotherapy by modulating the tumour microenvironment in a 4T1 orthotopic mouse model. Cancer Gene Ther. 2021. https://doi.org/10.1038/s41417-021-00389-3.
Ribas A, Dummer R, Puzanov I, VanderWalde A, Andtbacka RHI, Michielin O, Olszanski AJ, Malvehy J, Cebon J, Fernandez E, Kirkwood JM, Gajewski TF, Chen L, Gorski KS, Anderson AA, Diede SJ, Lassman ME, Gansert J, Hodi FS, Long GV. Oncolytic virotherapy promotes intratumoral T cell infiltration and improves anti-PD-1 immunotherapy. Cell. 2017;170(6):1109-19 e10. https://doi.org/10.1016/j.cell.2017.08.027.
Shafren DR, Dorahy DJ, Ingham RA, Burns GF, Barry RD. Coxsackievirus A21 binds to decay-accelerating factor but requires intercellular adhesion molecule 1 for cell entry. J Virol. 1997;71(6):4736–43. https://doi.org/10.1128/JVI.71.6.4736-4743.1997.
Miyamoto S, Inoue H, Nakamura T, Yamada M, Sakamoto C, Urata Y, Okazaki T, Marumoto T, Takahashi A, Takayama K, Nakanishi Y, Shimizu H, Tani K. Coxsackievirus B3 is an oncolytic virus with immunostimulatory properties that is active against lung adenocarcinoma. Cancer Res. 2012;72(10):2609–21. https://doi.org/10.1158/0008-5472.CAN-11-3185.
Janmaat ML, Rodriguez JA, Gallegos-Ruiz M, Kruyt FA, Giaccone G. Enhanced cytotoxicity induced by gefitinib and specific inhibitors of the Ras or phosphatidyl inositol-3 kinase pathways in non-small cell lung cancer cells. Int J Cancer. 2006;118(1):209–14. https://doi.org/10.1002/ijc.21290.
Guo WF, Lin RX, Huang J, Zhou Z, Yang J, Guo GZ, Wang SQ. Identification of differentially expressed genes contributing to radioresistance in lung cancer cells using microarray analysis. Radiat Res. 2005;164(1):27–35. https://doi.org/10.1667/rr3401.
Deng H, Liu H, de Silva T, Xue Y, Mohamud Y, Ng CS, Qu J, Zhang J, Jia WWG, Lockwood WW, Luo H. Coxsackievirus type B3 is a potent oncolytic virus against KRAS-mutant lung adenocarcinoma. Mol Ther Oncolytics. 2019;14:266–78. https://doi.org/10.1016/j.omto.2019.07.003.
Pandha H, Harrington K, Ralph C, Melcher A, Gupta S, Akerley W, Sandborn RE, Rudin C, Rosenberg J, Kaufman D, Schmidt E, Grose M, Shafren DR. Phase 1b KEYNOTE 200 (STORM study): A study of an intravenously delivered oncolytic virus, Coxsackievirus A21 in combination with pembrolizumab in advanced cancer patients [abstract]. Proceedings of the American Association for Cancer Research Annual Meeting 2017; 2017 Apr 1–5; Washington, DC Philadelphia (PA): AACR; Cancer Res 2017;77(13 Suppl):Abstract nr CT115
Rudin CM, Pandha HS, Gupta S, Zibelman MR, Akerley W, Day D, Hill AG, Sanborn RE, O’Day SJ, Clay TD, Wright GM, Jennens R, Gerber DE, Rosenberg JE, Ralph C, Campbell DC, Curti BD, Schmidt EV, Grose M, Shafen D. Phase Ib KEYNOTE-200: A study of an intravenously delivered oncolytic virus, coxsackievirus A21 in combination with pembrolizumab in advanced NSCLC and bladder cancer patients. Ann Oncol. 2018;29(8):VIII732.
Bischoff JR, Kirn DH, Williams A, Heise C, Horn S, Muna M, Ng L, Nye JA, Sampson-Johannes A, Fattaey A, McCormick F. An adenovirus mutant that replicates selectively in p53-deficient human tumor cells. Science. 1996;274(5286):373–6. https://doi.org/10.1126/science.274.5286.373.
Rao L, Debbas M, Sabbatini P, Hockenbery D, Korsmeyer S, White E. The adenovirus E1A proteins induce apoptosis, which is inhibited by the E1B 19-kDa and Bcl-2 proteins. Proc Natl Acad Sci U S A. 1992;89(16):7742–6. https://doi.org/10.1073/pnas.89.16.7742.
Nemunaitis J, Swisher SG, Timmons T, Connors D, Mack M, Doerksen L, Weill D, Wait J, Lawrence DD, Kemp BL, Fossella F, Glisson BS, Hong WK, Khuri FR, Kurie JM, Lee JJ, Lee JS, Nguyen DM, Nesbitt JC, Perez-Soler R, Pisters KM, Putnam JB, Richli WR, Shin DM, Walsh GL, Merritt J, Roth J. Adenovirus-mediated p53 gene transfer in sequence with cisplatin to tumors of patients with non-small-cell lung cancer. J Clin Oncol. 2000;18(3):609–22. https://doi.org/10.1200/JCO.2000.18.3.609.
Schuler M, Herrmann R, De Greve JL, Stewart AK, Gatzemeier U, Stewart DJ, Laufman L, Gralla R, Kuball J, Buhl R, Heussel CP, Kommoss F, Perruchoud AP, Shepherd FA, Fritz MA, Horowitz JA, Huber C, Rochlitz C. Adenovirus-mediated wild-type p53 gene transfer in patients receiving chemotherapy for advanced non-small-cell lung cancer: results of a multicenter phase II study. J Clin Oncol. 2001;19(6):1750–8. https://doi.org/10.1200/JCO.2001.19.6.1750.
Swisher SG, Roth JA, Nemunaitis J, Lawrence DD, Kemp BL, Carrasco CH, Connors DG, El-Naggar AK, Fossella F, Glisson BS, Hong WK, Khuri FR, Kurie JM, Lee JJ, Lee JS, Mack M, Merritt JA, Nguyen DM, Nesbitt JC, Perez-Soler R, Pisters KM, Putnam JB Jr, Richli WR, Savin M, Schrump DS, Shin DM, Shulkin A, Walsh GL, Wait J, Weill D, Waugh MK. Adenovirus-mediated p53 gene transfer in advanced non-small-cell lung cancer. J Natl Cancer Inst. 1999;91(9):763–71. https://doi.org/10.1093/jnci/91.9.763.
Guerrero C, Ensor JE, Sun K, Farach AM, Nair S, Zhang J, Singh M, Darcourt JG, Ramshesh PV, Butler EB, Teh BS, Sultenfuss M, Gupta N, Heslop HE, Mejia JA, Chang JC, Bernicker E. Stereotactic body radiation therapy and in situ oncolytic virus therapy followed by immunotherapy in metastatic non-small cell lung cancer. J Clin Oncol. 2021;39(15_suppl):9115.
Aggarwal C, Haas AR, Metzger S, Aguilar LK, Aguilar-Cordova E, Manzanera AG, Gomez-Hernandez G, Katz SI, Alley EW, Evans TL, Bauml JM, Cohen RB, Langer CJ, Albelda SM, Sterman DH. Phase I study of intrapleural gene-mediated cytotoxic immunotherapy in patients with malignant pleural effusion. Mol Ther. 2018;26(5):1198–205. https://doi.org/10.1016/j.ymthe.2018.02.015.
•• Schenk EL, Mandrekar SJ, Dy GK, Aubry MC, Tan AD, Dakhil SR, Sachs BA, Nieva JJ, Bertino E, Lee Hann C, Schild SE, Wadsworth TW, Adjei AA, Molina JR. A randomized double-blind phase II study of the Seneca Valley Virus (NTX-010) versus placebo for patients with extensive-stage SCLC (ES SCLC) who were stable or responding after at least four cycles of platinum-based chemotherapy: North Central Cancer Treatment Group (Alliance) N0923 Study. J Thorac Oncol. 2020;15(1):110–9. https://doi.org/10.1016/j.jtho.2019.09.083. This is one of the largest trials for the treatment of small cell lung cancer using oncolytic viruses.
Rudin CM, Poirier JT, Senzer NN, Stephenson J Jr, Loesch D, Burroughs KD, Reddy PS, Hann CL, Hallenbeck PL. Phase I clinical study of Seneca Valley Virus (SVV-001), a replication-competent picornavirus, in advanced solid tumors with neuroendocrine features. Clin Cancer Res. 2011;17(4):888–95. https://doi.org/10.1158/1078-0432.CCR-10-1706.
Bischoff JR, Samuel CE. Mechanism of interferon action. Activation of the human P1/eIF-2 alpha protein kinase by individual reovirus s-class mRNAs s1 mRNA is a potent activator relative to s4 mRNA. Virology. 1989;172(1):106–15. https://doi.org/10.1016/0042-6822(89)90112-8.
Strong JE, Coffey MC, Tang D, Sabinin P, Lee PW. The molecular basis of viral oncolysis: usurpation of the Ras signaling pathway by reovirus. EMBO J. 1998;17(12):3351–62. https://doi.org/10.1093/emboj/17.12.3351.
Sei S, Mussio JK, Yang QE, Nagashima K, Parchment RE, Coffey MC, Shoemaker RH, Tomaszewski JE. Synergistic antitumor activity of oncolytic reovirus and chemotherapeutic agents in non-small cell lung cancer cells. Mol Cancer. 2009;8:47. https://doi.org/10.1186/1476-4598-8-47.
Bradbury PA, Morris DG, Nicholas G, Tu D, Tehfe M, Goffin JR, Shepherd FA, Gregg RW, Rothenstein J, Lee C, Kuruvilla S, Keith BD, Torri V, Blais N, Hao D, Korpanty GJ, Goss G, Melosky BL, Mates M, Leighl N, Ayoub JP, Sederias J, Feilotter H, Seymour L, Laurie SA. Canadian Cancer Trials Group (CCTG) IND211: A randomized trial of pelareorep (Reolysin) in patients with previously treated advanced or metastatic non-small cell lung cancer receiving standard salvage therapy. Lung Cancer. 2018;120:142–8. https://doi.org/10.1016/j.lungcan.2018.03.005.
•• Villalona-Calero MA, Lam E, Otterson GA, Zhao W, Timmons M, Subramaniam D, Hade EM, Gill GM, Coffey M, Selvaggi G, Bertino E, Chao B, Knopp MV. Oncolytic reovirus in combination with chemotherapy in metastatic or recurrent non-small cell lung cancer patients with KRAS-activated tumors. Cancer. 2016;122(6):875–83. https://doi.org/10.1002/cncr.29856. This trial is an early-phase trial reporting results from combination oncolytic reovirus and chemotherapy for patients with non-small cell lung cancer.
Truong CS, Yoo SY. Oncolytic vaccinia virus in lung cancer vaccines. Vaccines (Basel). 2022;10(2). https://doi.org/10.3390/vaccines10020240.
Parato KA, Breitbach CJ, Le Boeuf F, Wang J, Storbeck C, Ilkow C, Diallo JS, Falls T, Burns J, Garcia V, Kanji F, Evgin L, Hu K, Paradis F, Knowles S, Hwang TH, Vanderhyden BC, Auer R, Kirn DH, Bell JC. The oncolytic poxvirus JX-594 selectively replicates in and destroys cancer cells driven by genetic pathways commonly activated in cancers. Mol Ther. 2012;20(4):749–58. https://doi.org/10.1038/mt.2011.276.
Cohen S, Kaufman HL. TG-4010 Transgene. Curr Opin Investig Drugs. 2004;5(12):1319–28.
Rochlitz C, Figlin R, Squiban P, Salzberg M, Pless M, Herrmann R, Tartour E, Zhao Y, Bizouarne N, Baudin M, Acres B. Phase I immunotherapy with a modified vaccinia virus (MVA) expressing human MUC1 as antigen-specific immunotherapy in patients with MUC1-positive advanced cancer. J Gene Med. 2003;5(8):690–9. https://doi.org/10.1002/jgm.397.
Ramlau R, Quoix E, Rolski J, Pless M, Lena H, Levy E, Krzakowski M, Hess D, Tartour E, Chenard MP, Limacher JM, Bizouarne N, Acres B, Halluard C, Velu T. A phase II study of Tg4010 (Mva-Muc1-Il2) in association with chemotherapy in patients with stage III/IV Non-small cell lung cancer. J Thorac Oncol. 2008;3(7):735–44. https://doi.org/10.1097/JTO.0b013e31817c6b4f.
Quoix E, Ramlau R, Westeel V, Papai Z, Madroszyk A, Riviere A, Koralewski P, Breton JL, Stoelben E, Braun D, Debieuvre D, Lena H, Buyse M, Chenard MP, Acres B, Lacoste G, Bastien B, Tavernaro A, Bizouarne N, Bonnefoy JY, Limacher JM. Therapeutic vaccination with TG4010 and first-line chemotherapy in advanced non-small-cell lung cancer: a controlled phase 2B trial. Lancet Oncol. 2011;12(12):1125–33. https://doi.org/10.1016/S1470-2045(11)70259-5.
•• Quoix E, Lena H, Losonczy G, Forget F, Chouaid C, Papai Z, Gervais R, Ottensmeier C, Szczesna A, Kazarnowicz A, Beck JT, Westeel V, Felip E, Debieuvre D, Madroszyk A, Adam J, Lacoste G, Tavernaro A, Bastien B, Halluard C, Palanche T, Limacher JM. TG4010 immunotherapy and first-line chemotherapy for advanced non-small-cell lung cancer (TIME): results from the phase 2b part of a randomised, double-blind, placebo-controlled, phase 2b/3 trial. Lancet Oncol. 2016;17(2):212–23. https://doi.org/10.1016/S1470-2045(15)00483-0. This trial is one of the largest phase III vaccine trial completed for the treatment of lung cancer.
Dash AS, Patel MR. Viroimmunotherapy of thoracic cancers. Biomedicines. 2017;5(1). https://doi.org/10.3390/biomedicines5010002
Reale A, Calistri A, Altomonte J. Giving oncolytic viruses a free ride: carrier cells for oncolytic virotherapy. pharmaceutics. 2021;13(12). https://doi.org/10.3390/pharmaceutics13122192.
Doronin K, Shashkova EV, May SM, Hofherr SE, Barry MA. Chemical modification with high molecular weight polyethylene glycol reduces transduction of hepatocytes and increases efficacy of intravenously delivered oncolytic adenovirus. Hum Gene Ther. 2009;20(9):975–88. https://doi.org/10.1089/hum.2009.028.
Green NK, Herbert CW, Hale SJ, Hale AB, Mautner V, Harkins R, Hermiston T, Ulbrich K, Fisher KD, Seymour LW. Extended plasma circulation time and decreased toxicity of polymer-coated adenovirus. Gene Ther. 2004;11(16):1256–63. https://doi.org/10.1038/sj.gt.3302295.
Hill C, Carlisle R. Achieving systemic delivery of oncolytic viruses. Expert Opin Drug Deliv. 2019;16(6):607–20. https://doi.org/10.1080/17425247.2019.1617269.
Liu XQ, Xin HY, Lyu YN, Ma ZW, Peng XC, Xiang Y, Wang YY, Wu ZJ, Cheng JT, Ji JF, Zhong JX, Ren BX, Wang XW, Xin HW. Oncolytic herpes simplex virus tumor targeting and neutralization escape by engineering viral envelope glycoproteins. Drug Deliv. 2018;25(1):1950–62. https://doi.org/10.1080/10717544.2018.1534895.
Bommareddy PK, Shettigar M, Kaufman HL. Integrating oncolytic viruses in combination cancer immunotherapy. Nat Rev Immunol. 2018;18(8):498–513. https://doi.org/10.1038/s41577-018-0014-6.
Li L, Liu S, Han D, Tang B, Ma J. Delivery and biosafety of oncolytic virotherapy. Front Oncol. 2020;10:475. https://doi.org/10.3389/fonc.2020.00475.
Russell SJ, Barber GN. Oncolytic viruses as antigen-agnostic cancer vaccines. Cancer Cell. 2018;33(4):599–605. https://doi.org/10.1016/j.ccell.2018.03.011.
Li J, O’Malley M, Urban J, Sampath P, Guo ZS, Kalinski P, Thorne SH, Bartlett DL. Chemokine expression from oncolytic vaccinia virus enhances vaccine therapies of cancer. Mol Ther. 2011;19(4):650–7. https://doi.org/10.1038/mt.2010.312.
Lu S, Zhang Z, Du P, Chard LS, Yan W, El Khouri M, Wang Z, Zhang Z, Chu Y, Gao D, Zhang Q, Zhang L, Nagano A, Wang J, Chelala C, Liu J, Chen J, Liu P, Dong Y, Wang S, Li X, Dong J, Lemoine NR, Pei D, Wang Y. A virus-infected, reprogrammed somatic cell-derived tumor cell (VIReST) vaccination regime can prevent initiation and progression of pancreatic cancer. Clin Cancer Res. 2020;26(2):465–76. https://doi.org/10.1158/1078-0432.CCR-19-1395.
Tysome JR, Li X, Wang S, Wang P, Gao D, Du P, Chen D, Gangeswaran R, Chard LS, Yuan M, Alusi G, Lemoine NR, Wang Y. A novel therapeutic regimen to eradicate established solid tumors with an effective induction of tumor-specific immunity. Clin Cancer Res. 2012;18(24):6679–89. https://doi.org/10.1158/1078-0432.CCR-12-0979.
Nishio N, Diaconu I, Liu H, Cerullo V, Caruana I, Hoyos V, Bouchier-Hayes L, Savoldo B, Dotti G. Armed oncolytic virus enhances immune functions of chimeric antigen receptor-modified T cells in solid tumors. Cancer Res. 2014;74(18):5195–205. https://doi.org/10.1158/0008-5472.CAN-14-0697.
Suryadevara CM, Gedeon PC, Sanchez-Perez L, Verla T, Alvarez-Breckenridge C, Choi BD, Fecci PE, Sampson JH. Are BiTEs the “missing link” in cancer therapy? Oncoimmunology. 2015;4(6):e1008339. https://doi.org/10.1080/2162402X.2015.1008339.
Wing A, Fajardo CA, Posey AD Jr, Shaw C, Da T, Young RM, Alemany R, June CH, Guedan S. Improving CART-cell therapy of solid tumors with oncolytic virus-driven production of a bispecific T-cell engager. Cancer Immunol Res. 2018;6(5):605–16. https://doi.org/10.1158/2326-6066.CIR-17-0314.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Jyoti Malhotra has served on the advisory board for Astra-Zeneca, Blueprint Medicines, Mirati Therapeutics, Sanofi, Oncocyte, and Beigene and received research funding from Bristol-Myers Squibb, Celldex, Biohaven, Daiichi Sankyo, and Beyond Spring Pharmaceuticals. Edward Kim has received personal fees from AstraZeneca and Genentech.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Lung Cancer
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Malhotra, J., Kim, E.S. Oncolytic Viruses and Cancer Immunotherapy. Curr Oncol Rep 25, 19–28 (2023). https://doi.org/10.1007/s11912-022-01341-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11912-022-01341-w