Abstract
Purpose of Review
This article serves to describe recent controversies in cancer prehabilitation including efficacy, dose, cost effectiveness, stakeholder input, and international implementation.
Recent Findings
Appropriate frequency, type, and timing have yet to be determined, but high intensity exercise is recommended. Costs are favorable when modeled and information on costs of real-world application are forthcoming. Patients are interested in and willing to attend cancer prehabilitation. Cancer prehabilitation research is spreading throughout the world.
Summary
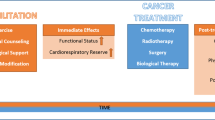
Cancer prehabilitation includes assessment of a newly diagnosed cancer patient’s baseline fitness and targeted interventions to improve their health before surgery, chemotherapy, or radiation. Cancer prehabilitation improves fitness as measured preoperatively and improves outcomes postoperatively.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
• Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49. This article provides an update on the global cancer burden.
Silver JK, Baima J, Mayer RS. Impairment-driven cancer rehabilitation: an essential component of quality care and survivorship. CA Cancer J Clin. 2013;63(5):295–317.
Carli F, Silver JK, Feldman LS, McKee A, Gilman S, Gillis C, et al. Surgical prehabilitation in patients with cancer: state-of-the-science and recommendations for future research from a panel of subject matter experts. Phys Med Rehabil Clin N Am. 2017;28(1):49–64.
Silver JK, Baima J. Cancer prehabilitation: an opportunity to decrease treatment-related morbidity, increase cancer treatment options, and improve physical and psychological health outcomes. Am J Phys Med Rehabil. 2013;92(8):715–27.
International Prehabilitation Society 2019 [October 25, 2021]. Available from: https://prehabsociety.com/activities/.
Snyder AR, Parsons JT, Valovich McLeod TC, Curtis Bay R, Michener LA, Sauers EL. Using disablement models and clinical outcomes assessment to enable evidence-based athletic training practice, part I: disablement models. J Athl Train. 2008;43(4):428–36.
Levangie PK, Santasier AM, Stout NL, Pfalzer L. A qualitative assessment of upper quarter dysfunction reported by physical therapists treated for breast cancer or treating breast cancer sequelae. Support Care Cancer. 2011;19(9):1367–78.
Gillis C, Buhler K, Bresee L, Carli F, Gramlich L, Culos-Reed N, et al. Effects of nutritional prehabilitation, with and without exercise, on outcomes of patients who undergo colorectal surgery: a systematic review and meta-analysis. Gastroenterology. 2018;155(2):391-410.e4.
•• Daniels SL, Lee MJ, George J, Kerr K, Moug S, Wilson TR, et al. Prehabilitation in elective abdominal cancer surgery in older patients: systematic review and meta-analysis. BJS Open. 2020;4(6):1022–41. This study, which included an older patient population, demonstrated an improvement in the multimodal prehabilitation group compared to baseline for 6MWT and a reduction in postoperative complications that was not seen in the unimodal group.
Moran J, Guinan E, McCormick P, Larkin J, Mockler D, Hussey J, et al. The ability of prehabilitation to influence postoperative outcome after intra-abdominal operation: a systematic review and meta-analysis. Surgery. 2016;160(5):1189–201.
•• Michael CM, Lehrer EJ, Schmitz KH, Zaorsky NG. Prehabilitation exercise therapy for cancer: a systematic review and meta-analysis. Cancer Med. 2021;10(13):4195–205. The meta-analysis demonstrated an improvement in 6MWD in prehabilitation compared to the control group at 4 to 8 weeks postoperatively.
•• Waterland JL, McCourt O, Edbrooke L, Granger CL, Ismail H, Riedel B, et al. Efficacy of prehabilitation including exercise on postoperative outcomes following abdominal cancer surgery: a systematic review and meta-analysis. Front Surg. 2021;8:628848. A point of strength of this study is the variability of subject groups, including approximately 12 cancers pathologies in 14 countries. Meta-analysis demonstrated that 6MWT was improved in multimodal prehabilitation whereas unimodal exercise-only did not achieve significance.
•• Rosero ID, Ramírez-Vélez R, Lucia A, Martínez-Velilla N, Santos-Lozano A, Valenzuela PL, et al. Systematic review and meta-analysis of randomized, controlled trials on preoperative physical exercise interventions in patients with non-small-cell lung cancer. Cancers (Basel). 2019;11(7). This was the only study to include exclusively NSCLC patients. A significant difference in difference in 6MWT, VO2peak, and a reduction in postoperative hospitalizations were found between the prehabilitation and usual care groups.
•• Lambert JE, Hayes LD, Keegan TJ, Subar DA, Gaffney CJ. The impact of prehabilitation on patient outcomes in hepatobiliary, colorectal, and upper gastrointestinal cancer surgery: a PRISMA-accordant meta-analysis. Ann Surg. 2021;274(1):70–7. This is the only study included that analyzed 6MWT but found no significant improvement with prehabilitation.
Levett DZH, Jack S, Swart M, Carlisle J, Wilson J, Snowden C, et al. Perioperative cardiopulmonary exercise testing (CPET): consensus clinical guidelines on indications, organization, conduct, and physiological interpretation. Br J Anaesth. 2018;120(3):484–500.
•• Palma S, Hasenoehrl T, Jordakieva G, Ramazanova D, Crevenna R. High-intensity interval training in the prehabilitation of cancer patients-a systematic review and meta-analysis. Support Care Cancer. 2021;29(4):1781–94. In this study prehabilitation HIIT improved VO2 peak prior to surgery versus usual care. Participant adherence was analyzed and revealed as the intervention period increased, the participant adherence declined.
•• Smyth E, O'Connor L, Mockler D, Reynolds JV, Hussey J, Guinan E. Preoperative high intensity interval training for oncological resections: a systematic review and meta-analysis. Surg Oncol. 2021;38:101620. In this study there was insufficient evidence to show that HIIT improved VO2 peak prior to surgery.
Treanor C, Kyaw T, Donnelly M. An international review and meta-analysis of prehabilitation compared to usual care for cancer patients. J Cancer Surviv. 2018;12(1):64–73.
Benzo R, Wigle D, Novotny P, Wetzstein M, Nichols F, Shen RK, et al. Preoperative pulmonary rehabilitation before lung cancer resection: results from two randomized studies. Lung Cancer. 2011;74(3):441–5.
Sebio Garcia R, Yáñez Brage MI, Giménez Moolhuyzen E, Granger CL, Denehy L. Functional and postoperative outcomes after preoperative exercise training in patients with lung cancer: a systematic review and meta-analysis. Interact Cardiovasc Thorac Surg. 2016;23(3):486–97.
•• Stover AM, Kurtzman R, Walker Bissram J, Jansen J, Carr P, Atkinson T, et al. Stakeholder perceptions of key aspects of high-quality cancer care to assess with patient reported outcome measures: a systematic review. Cancers (Basel). 2021;13(14). This study identfied stakeholder opinions on the most important aspects of high-quality cancer care.
Wu F, Laza-Cagigas R, Pagarkar A, Olaoke A, El Gammal M, Rampal T. The feasibility of prehabilitation as part of the breast cancer treatment pathway. Pm r. 2021;13(11):1237–46.
• Singh B, Spence R, Steele ML, Hayes S, Toohey K. Exercise for individuals with lung cancer: a systematic review and meta-analysis of adverse events, feasibility, and effectiveness. Semin Oncol Nurs. 2020;36(5):151076. This study commented on patient adherence and satisfaction with safety and effectiveness of prehabilitation intervention.
Jones LW, Courneya KS. Exercise counseling and programming preferences of cancer survivors. Cancer Pract. 2002;10(4):208–15.
Barberan-Garcia A, Navarro-Ripoll R, Sánchez-Lorente D, Moisés-Lafuente J, Boada M, Messaggi-Sartor M, et al. Cost-effectiveness of a technology-supported multimodal prehabilitation program in moderate-to-high risk patients undergoing lung cancer resection: randomized controlled trial protocol. BMC Health Serv Res. 2020;20(1):207.
van Rooijen S, Carli F, Dalton S, Thomas G, Bojesen R, Le Guen M, et al. Multimodal prehabilitation in colorectal cancer patients to improve functional capacity and reduce postoperative complications: the first international randomized controlled trial for multimodal prehabilitation. BMC Cancer. 2019;19(1):98.
• Ploussard G, Almeras C, Beauval JB, Gautier JR, Garnault V, Frémont N, et al. A combination of enhanced recovery after surgery and prehabilitation pathways improves perioperative outcomes and costs for robotic radical prostatectomy. Cancer. 2020;126(18):4148–55. This article demonstarted that addition of prehabilitation to an enhanced recovery pathway (ERAS) in patients undergoing robot assisted radical prostatectomy was cost-saving.
• Dholakia J, Cohn DE, Straughn JM, Dilley SE. Prehabilitation for medically frail patients undergoing surgery for epithelial ovarian cancer: a cost-effectiveness analysis. J Gynecol Oncol. 2021;32(6):0. This is a recent analysis of prehabilitation versus usual care in medically frail that found prehabilitation to be cost-saving, which continues to be a point of interest in the implementation of prehabilitation programs.
• Howard R, Yin YS, McCandless L, Wang S, Englesbe M, Machado-Aranda D. Taking control of your surgery: impact of a prehabilitation program on major abdominal surgery. J Am Coll Surg. 2019;228(1):72–80. This artcle demonstrated that home-based technology-focused prehabilitation has high levels of partient satisfaction and compliance while being cost-effective.
Kiss N, Baguley BJ, Ball K, Daly RM, Fraser SF, Granger CL, et al. Technology-supported self-guided nutrition and physical activity interventions for adults with cancer: systematic review. JMIR Mhealth Uhealth. 2019;7(2):e12281.
• Koh FH, Loh CH, Tan WJ, Ho LML, Yen D, Chua JMW, et al. Structured presurgery prehabilitation for aged patients undergoing elective surgery significantly improves surgical outcomes and reduces cost: a nonrandomized sequential comparative prospective cohort study. Nutr Clin Pract. 2021. This study reported significant savings via reduction in hospital length of stay.
• Akiyama Y, Sasaki A, Fujii Y, Fujisawa R, Sasaki N, Nikai H, et al. Efficacy of enhanced prehabilitation for patients with esophageal cancer undergoing esophagectomy. Esophagus. 2021;18(1):56–64. This study demonstrates as example of emerging prehabilitation literature out of Japan.
• Lee S-H, Lee N-R, Kim J-W, Lee M, Seong S-J, Song J-Y, et al. Feasibility and acceptability of prehabilitation before surgery for endometrial cancer. KJSM. 2020;38(2):85–94. This study demonstrates as example of emerging prehabilitation literature out of Korea.
• Shun SC. Proposing a comprehensive prehabilitation model for individuals with operable pancreatic cancer. Asia Pac J Oncol Nurs. 2020;7(3):255–8. This study demonstrates as example of emerging prehabilitation literature out of Taiwan.
• Swaminathan N, Kundra P, Ravi R, Kate V. ERAS protocol with respiratory prehabilitation versus conventional perioperative protocol in elective gastrectomy - a randomized controlled trial. Int J Surg. 2020;81:149–57. This study demonstrates as example of emerging prehabilitation literature out of India.
• Zhou YB. [Prehabilitation for gastrointestinal cancer patients]. Zhonghua Wei Chang Wai Ke Za Zhi. 2021;24(2):122–7. This study demonstrates as example of emerging prehabilitation literature out of China.
•• World Health Organization: World Health Organization; [3 February 2022:[Available from: https://www.who.int/news-room/fact-sheets/detail/cancer. WHO reports low- and middle-income countries account for a disproportionate percentage of cancer deaths worldwide with low treatment availability.
WHO Guidelines Approved by the Guidelines Review Committee. World Report on Disability 2011. Geneva: World Health Organization Copyright © World Health Organization 2011.; 2011.
Fulop A, Lakatos L, Susztak N, Szijarto A, Banky B. The effect of trimodal prehabilitation on the physical and psychological health of patients undergoing colorectal surgery: a randomised clinical trial. Anaesthesia. 2021;76(1):82–90.
• Fernandes ADV, Moreira-Gonçalves D, Come J, Rosa NC, Costa V, Lopes LV, et al. Prehabilitation program for African sub-Saharan surgical patients is an unmet need. Pan Afr Med J. 2020;36:62. This study demonstrates the unmet need for prehabilitation in Africa given known positive impact on surgical recovery.
• Baima J. "Prehabilitation" in Lung Cancer Rehabilitation. Ed. Adrian Christian.1st. ed 2022. p. 121. This publication provides an example lung cancer prehabilitation program.
• Smith SR, Vargo M, Zucker DS, Henderson M, Shahpar S, Wisotzky EM, et al. The cancer rehabilitation medicine metrics consortium: a path to enhanced, multi-site outcome assessment to enhance care and demonstrate value. Front Oncol. 2020;10:625700. This paper explains the Cancer Rehabilitation Medicine Metrics Consortium's process and conclusions in developing an assessment tool to evaluate function in cancer patients.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors have no competing interests to report.
Human and Animal Rights and Informed Consent
All reported studies/experiments with human or animal subjects performed by the authors have been previously published and complied with all applicable ethical standards (including the Helsinki declaration and its amendments, institutional/national research committee standards, and international/national/institutional guidelines).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Cancer Rehabilitation
Rights and permissions
About this article
Cite this article
Coderre, D., Brahmbhatt, P., Hunter, T.L. et al. Cancer Prehabilitation in Practice: the Current Evidence. Curr Oncol Rep 24, 1569–1577 (2022). https://doi.org/10.1007/s11912-022-01304-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11912-022-01304-1