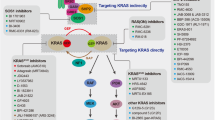
Abstract
Purpose of Review
Mutations in kirsten rat sarcoma viral oncogene homolog (KRAS) are the most frequently observed genomic alterations in human cancers. No KRAS targeting therapy has been approved despite more than three decades of efforts. Encouraging progress has been made in targeting KRASG12C with KRASG12C specific covalent inhibitors in the past few years. Herein, we review the recent breakthroughs in KRAS targeting.
Recent Findings
KRASG12C mutation was found in 14% of non-small cell lung cancer (NSCLC) and 3% of colorectal cancer. Recently, highly potent KRASG12C specific inhibitors have been developed and demonstrated potent activity in preclinical models. Early results from phase 1 clinical trials with sotorasib and MRTX849 show promising antitumor activity in NSCLC, colorectal cancer and other solid tumors harboring KRASG12C mutation.
Summary
For the first time, the preclinical success of targeting KRAS has translated into clinical benefits, which holds the potential of transforming clinical management of KRAS mutated solid tumors. Additional efforts are needed to identify biomarkers that predict response to KRAS inhibition in patients with KRASG12C as well as to develop strategies to overcome resistance.
Similar content being viewed by others
Data Availability
Not applicable.
References
Papers of particular interest, published recently, have been highlighted as: •• Of major importance
DeSantis CE, Ma J, Gaudet MM, Newman LA, Miller KD, Goding Sauer A, et al. Breast cancer statistics, 2019. CA Cancer J Clin. 2019;69(6):438–51. https://doi.org/10.3322/caac.21583.
Qin S, Li J, Wang L, Xu J, Cheng Y, Bai Y, et al. Efficacy and tolerability of first-line Cetuximab plus Leucovorin, fluorouracil, and Oxaliplatin (FOLFOX-4) versus FOLFOX-4 in patients with RAS wild-type metastatic colorectal Cancer: the open-label, randomized, phase III TAILOR trial. J Clin Oncol. 2018;36(30):3031–9. https://doi.org/10.1200/jco.2018.78.3183.
Cutsem EV, Lenz H-J, Köhne C-H, Heinemann V, Tejpar S, Melezínek I, et al. Fluorouracil, Leucovorin, and Irinotecan plus Cetuximab treatment and RAS mutations in colorectal Cancer. J Clin Oncol. 2015;33(7):692–700. https://doi.org/10.1200/jco.2014.59.4812.
Douillard J-Y, Oliner KS, Siena S, Tabernero J, Burkes R, Barugel M, et al. Panitumumab–FOLFOX4 treatment and RAS mutations in colorectal Cancer. N Engl J Med. 2013;369(11):1023–34. https://doi.org/10.1056/NEJMoa1305275.
Siegel RL, Miller KD, Goding Sauer A, Fedewa SA, Butterly LF, Anderson JC, et al. Colorectal cancer statistics, 2020. CA Cancer J Clin. 2020;70:145–64. https://doi.org/10.3322/caac.21601.
Cremolini C, Loupakis F, Antoniotti C, Lupi C, Sensi E, Lonardi S, et al. FOLFOXIRI plus bevacizumab versus FOLFIRI plus bevacizumab as first-line treatment of patients with metastatic colorectal cancer: updated overall survival and molecular subgroup analyses of the open-label, phase 3 TRIBE study. The Lancet Oncology. 2015;16(13):1306–15. https://doi.org/10.1016/S1470-2045(15)00122-9.
Grothey A, Van Cutsem E, Sobrero A, Siena S, Falcone A, Ychou M, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381(9863):303–12. https://doi.org/10.1016/S0140-6736(12)61900-X.
Mayer RJ, Van Cutsem E, Falcone A, Yoshino T, Garcia-Carbonero R, Mizunuma N, et al. Randomized trial of TAS-102 for refractory metastatic colorectal Cancer. N Engl J Med. 2015;372(20):1909–19. https://doi.org/10.1056/NEJMoa1414325.
Karapetis CS, Khambata-Ford S, Jonker DJ, O'Callaghan CJ, Tu D, Tebbutt NC, et al. K-ras mutations and benefit from Cetuximab in advanced colorectal Cancer. N Engl J Med. 2008;359(17):1757–65. https://doi.org/10.1056/NEJMoa0804385.
Amado RG, Wolf M, Peeters M, Van Cutsem E, Siena S, Freeman DJ, et al. Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008;26(10):1626–34. https://doi.org/10.1200/jco.2007.14.7116.
Van Cutsem E, Cervantes A, Adam R, Sobrero A, Van Krieken JH, Aderka D, et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Annals of oncology : official journal of the European Society for Medical Oncology. 2016;27(8):1386–422. https://doi.org/10.1093/annonc/mdw235.
Taieb J, Le Malicot K, Shi Q, Penault-Llorca F, Bouché O, Tabernero J et al. Prognostic Value of BRAF and KRAS Mutations in MSI and MSS Stage III Colon Cancer. Journal of the National Cancer Institute. 2017;109(5). https://doi.org/10.1093/jnci/djw272.
Holch JW, Ricard I, Stintzing S, Modest DP, Heinemann V. The relevance of primary tumour location in patients with metastatic colorectal cancer: A meta-analysis of first-line clinical trials. European journal of cancer (Oxford, England : 1990). 2017;70:87–98. https://doi.org/10.1016/j.ejca.2016.10.007.
Sanchez-Vega F, Mina M, Armenia J, Chatila WK, Luna A, La KC et al. Oncogenic Signaling Pathways in The Cancer Genome Atlas. Cell. 2018;173(2):321–37.e10. https://doi.org/10.1016/j.cell.2018.03.035.
Ou SHI, Sokol ES, Madison R, Chung J, Ross JS, Miller VA, et al. 92PD - comprehensive pan-cancer analysis of KRAS genomic alterations (GA) including potentially targetable subsets. Ann Oncol. 2019;30:v26. https://doi.org/10.1093/annonc/mdz239.003.
Neumann J, Zeindl-Eberhart E, Kirchner T, Jung A. Frequency and type of KRAS mutations in routine diagnostic analysis of metastatic colorectal cancer. Pathol Res Pract. 2009;205(12):858–62. https://doi.org/10.1016/j.prp.2009.07.010.
Ouerhani S, Elgaaied AB. The mutational spectrum of HRAS, KRAS, NRAS and FGFR3 genes in bladder cancer. Cancer biomarkers : section A of Disease markers. 2011;10(6):259–66. https://doi.org/10.3233/cbm-2012-0254.
Muñoz-Maldonado C, Zimmer Y, Medová M. A Comparative Analysis of Individual RAS Mutations in Cancer Biology. Front Oncol. 2019;9:1088. https://doi.org/10.3389/fonc.2019.01088.
Khan AQ, Kuttikrishnan S, Siveen KS, Prabhu KS, Shanmugakonar M, Al-Naemi HA, et al. RAS-mediated oncogenic signaling pathways in human malignancies. Semin Cancer Biol. 2019;54:1–13. https://doi.org/10.1016/j.semcancer.2018.03.001.
McCormick F. Targeting KRAS directly. Annual Review of Cancer Biology. 2018;2(1):81–90. https://doi.org/10.1146/annurev-cancerbio-050216-122010.
Ostrem JM, Peters U, Sos ML, Wells JA, Shokat KM. K-Ras(G12C) inhibitors allosterically control GTP affinity and effector interactions. Nature. 2013;503(7477):548–51. https://doi.org/10.1038/nature12796.
Canon J, Rex K, Saiki AY, Mohr C, Cooke K, Bagal D et al. The clinical KRAS(G12C) inhibitor AMG 510 drives anti-tumour immunity. Nature. 2019;575(7781):217–23. https://doi.org/10.1038/s41586-019-1694-1. In preclincal setting, AMG510 led to regression of tumors harboring KRASG12C mutation. The improved anti-tumor activity with AMG510 in combination with targeted therapies such as EGFR, AKT and MEK suggests that combining AMG510 with MAPK targeting agents may lead to improved efficacy in clinical setting.
Hallin J, Engstrom LD, Hargis L, Calinisan A, Aranda R, Briere DM, et al. The KRAS<sup>G12C</sup> inhibitor MRTX849 provides insight toward therapeutic susceptibility of KRAS-mutant cancers in mouse models and patients. Cancer Discovery. 2020;10(1):54–71. https://doi.org/10.1158/2159-8290.cd-19-1167.
Fakih M, O'Neil B, Price TJ, Falchook GS, Desai J, Kuo J et al. Phase 1 study evaluating the safety, tolerability, pharmacokinetics (PK), and efficacy of AMG 510, a novel small molecule KRASG12C inhibitor, in advanced solid tumors. Journal of Clinical Oncology. 2019;37(15_suppl):3003. https://doi.org/10.1200/JCO.2019.37.15_suppl.3003.
Fakih M, Desai J, Kuboki Y, Strickler JH, Price TJ, Durm GA et al. CodeBreak 100: Activity of AMG 510, a novel small molecule inhibitor of KRASG12C, in patients with advanced colorectal cancer. Journal of Clinical Oncology. 2020;38(15_suppl):4018. https://doi.org/10.1200/JCO.2020.38.15_suppl.4018.
Hong DS, Kuo J, Sacher AG, Barlesi F, Besse B, Kuboki Y et al. CodeBreak 100: Phase I study of AMG 510, a novel KRASG12C inhibitor, in patients (pts) with advanced solid tumors other than non-small cell lung cancer (NSCLC) and colorectal cancer (CRC). Journal of Clinical Oncology. 2020;38(15_suppl):3511. https://doi.org/10.1200/JCO.2020.38.15_suppl.3511.
Rajalingam K, Schreck R, Rapp UR, Albert S. Ras oncogenes and their downstream targets. Bba-Mol Cell Res. 2007;1773(8):1177–95. https://doi.org/10.1016/j.bbamcr.2007.01.012.
Pylayeva-Gupta Y, Grabocka E, Bar-Sagi D. RAS oncogenes: weaving a tumorigenic web. Nat Rev Cancer. 2011;11(11):761–74. https://doi.org/10.1038/nrc3106.
Simanshu DK, Nissley DV, McCormick F. RAS proteins and their regulators in human disease. Cell. 2017;170(1):17–33. https://doi.org/10.1016/j.cell.2017.06.009.
Bos JL, Rehmann H, Wittinghofer A. GEFs and GAPs: critical elements in the control of small G proteins. Cell. 2007;129(5):865–77. https://doi.org/10.1016/j.cell.2007.05.018.
Downward J. Targeting RAS signalling pathways in cancer therapy. Nat Rev Cancer. 2003;3(1):11–22. https://doi.org/10.1038/nrc969.
Gibbs JB, Sigal IS, Poe M, Scolnick EM. Intrinsic GTPase activity distinguishes normal and oncogenic ras p21 molecules. Proc Natl Acad Sci. 1984;81(18):5704–8. https://doi.org/10.1073/pnas.81.18.5704.
John J, Sohmen R, Feuerstein J, Linke R, Wittinghofer A, Goody RS. Kinetics of interaction of nucleotides with nucleotide-free H-ras p21. Biochemistry. 1990;29(25):6058–65. https://doi.org/10.1021/bi00477a025.
Cox AD, Fesik SW, Kimmelman AC, Luo J, Der CJ. Drugging the undruggable RAS: Mission possible? Nat Rev Drug Discov. 2014;13(11):828–51. https://doi.org/10.1038/nrd4389.
Lazo JS, Sharlow ER. Drugging Undruggable molecular Cancer targets. Annu Rev Pharmacol Toxicol. 2016;56:23–40. https://doi.org/10.1146/annurev-pharmtox-010715-103440.
Reiss Y, Goldstein JL, Seabra MC, Casey PJ, Brown MS. Inhibition of purified p21ras farnesyl:protein transferase by Cys-AAX tetrapeptides. Cell. 1990;62(1):81–8. https://doi.org/10.1016/0092-8674(90)90242-7.
Whyte DB, Kirschmeier P, Hockenberry TN, Nunez-Oliva I, James L, Catino JJ, et al. K- and N-Ras are geranylgeranylated in cells treated with farnesyl protein transferase inhibitors. J Biol Chem. 1997;272(22):14459–64. https://doi.org/10.1074/jbc.272.22.14459.
Rowell CA, Kowalczyk JJ, Lewis MD, Garcia AM. Direct demonstration of geranylgeranylation and farnesylation of Ki-Ras in vivo. J Biol Chem. 1997;272(22):14093–7. https://doi.org/10.1074/jbc.272.22.14093.
End DW, Smets G, Todd AV, Applegate TL, Fuery CJ, Angibaud P, et al. Characterization of the antitumor effects of the selective farnesyl protein transferase inhibitor R115777 in vivo and in vitro. Cancer Res. 2001;61(1):131–7.
Van Cutsem E, van de Velde H, Karasek P, Oettle H, Vervenne WL, Szawlowski A, et al. Phase III trial of gemcitabine plus tipifarnib compared with gemcitabine plus placebo in advanced pancreatic cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2004;22(8):1430–8. https://doi.org/10.1200/jco.2004.10.112.
Rao S, Cunningham D, de Gramont A, Scheithauer W, Smakal M, Humblet Y, et al. Phase III double-blind placebo-controlled study of farnesyl transferase inhibitor R115777 in patients with refractory advanced colorectal cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2004;22(19):3950–7. https://doi.org/10.1200/jco.2004.10.037.
Ostrem JM, Peters U, Sos ML, Wells JA, Shokat KM. K-Ras(G12C) inhibitors allosterically control GTP affinity and effector interactions. Nature. 2013;503(7477):548. https://doi.org/10.1038/nature12796.
Lito P, Solomon M, Li L-S, Hansen R, Rosen N. Allele-specific inhibitors inactivate mutant KRAS G12C by a trapping mechanism. Science. 2016;351(6273):604–8. https://doi.org/10.1126/science.aad6204.
Patricelli MP, Janes MR, Li L-S, Hansen R, Peters U, Kessler LV, et al. Selective inhibition of oncogenic KRAS output with small molecules targeting the inactive state. Cancer Discovery. 2016;6(3):316–29. https://doi.org/10.1158/2159-8290.cd-15-1105.
Janes MR, Zhang J, Li LS, Hansen R, Peters U, Guo X et al. Targeting KRAS Mutant Cancers with a Covalent G12C-Specific Inhibitor. Cell. 2018;172(3):578–89.e17. :https://doi.org/10.1016/j.cell.2018.01.006.
Gentile DR, Rathinaswamy MK, Jenkins ML, Moss SM, Siempelkamp BD, Renslo AR et al. Ras Binder Induces a Modified Switch-II Pocket in GTP and GDP States. Cell Chem Biol. 2017;24(12):1455. https://doi.org/10.1016/j.chembiol.2017.08.025.
Saiki AY, Gaida K, Rex K, Achanta P, Miguel TS, Koppada N et al. Abstract 4484: Discovery and in vitro characterization of AMG 510–a potent and selective covalent small-molecule inhibitor of KRAS<sup>G12C</sup>. Cancer research. 2019;79(13 Supplement):4484-. doi:https://doi.org/10.1158/1538-7445.am2019-4484.
Hong DS, Fakih MG, Strickler JH, Desai J, Durm GA, Shapiro GI et al. KRASG12C Inhibition with Sotorasib in Advanced Solid Tumors. New England Journal of Medicine. 2020. doi:https://doi.org/10.1056/NEJMoa1917239. This phase 1 clinical trial showed promising activity of sotorasib in patients with KRASG12C mutated solid tumors, such as NSCLC (ORR, 32.2%; DCR, 88.1%) and colorectal cancer (ORR, 7.1%; DCR 73.8%).
Robert C, Grob JJ, Stroyakovskiy D, Karaszewska B, Hauschild A, Levchenko E, et al. Five-year outcomes with Dabrafenib plus Trametinib in metastatic melanoma. N Engl J Med. 2019;381(7):626–36. https://doi.org/10.1056/NEJMoa1904059.
Corcoran RB, André T, Atreya CE, Schellens JHM, Yoshino T, Bendell JC, et al. Combined BRAF, EGFR, and MEK inhibition in patients with <em>BRAF</em><sup>V600E</sup>−mutant colorectal Cancer. Cancer Discovery. 2018;8(4):428–43. https://doi.org/10.1158/2159-8290.cd-17-1226.
Planchard D, Besse B, Groen HJM, Souquet PJ, Quoix E, Baik CS, et al. Dabrafenib plus trametinib in patients with previously treated BRAF(V600E)-mutant metastatic non-small cell lung cancer: an open-label, multicentre phase 2 trial. The Lancet Oncology. 2016;17(7):984–93. https://doi.org/10.1016/s1470-2045(16)30146-2.
Planchard D, Smit EF, Groen HJM, Mazieres J, Besse B, Helland Å, et al. Dabrafenib plus trametinib in patients with previously untreated BRAFV600E-mutant metastatic non-small-cell lung cancer: an open-label, phase 2 trial. The Lancet Oncology. 2017;18(10):1307–16. https://doi.org/10.1016/S1470-2045(17)30679-4.
Tran E, Robbins PF, Lu Y-C, Prickett TD, Gartner JJ, Jia L, et al. T-cell transfer therapy targeting mutant KRAS in Cancer. N Engl J Med. 2016;375(23):2255–62. https://doi.org/10.1056/NEJMoa1609279.
Tran E, Ahmadzadeh M, Lu Y-C, Gros A, Turcotte S, Robbins PF, et al. Immunogenicity of somatic mutations in human gastrointestinal cancers. Science. 2015;350(6266):1387–90. https://doi.org/10.1126/science.aad1253.
Cafri G, Yossef R, Pasetto A, Deniger DC, Lu Y-C, Parkhurst M, et al. Memory T cells targeting oncogenic mutations detected in peripheral blood of epithelial cancer patients. Nat Commun. 2019;10(1):449. https://doi.org/10.1038/s41467-019-08304-z.
Lu Y, Bellgrau D, Dwyer-Nield LD, Malkinson AM, Duke RC, Rodell TC, et al. Mutation-selective tumor remission with Ras-targeted. Whole Yeast-Based Immunotherapy Cancer research. 2004;64(15):5084–8. https://doi.org/10.1158/0008-5472.can-04-1487.
Cohn A, Morse MA, O’Neil B, Bellgrau D, Duke RC, Franzusoff AJ et al. Treatment of Ras mutation-bearing solid tumors using whole recombinant S. cerevisiae yeast expressing mutated Ras: Preliminary safety and immunogenicity results from a phase 1 trial. Journal of Clinical Oncology. 2005;23(16_suppl):2571. https://doi.org/10.1200/jco.2005.23.16_suppl.2571.
Muscarella P, Wilfong LS, Ross SB, Richards DA, Raynov J, Fisher WE et al. A randomized, placebo-controlled, double blind, multicenter phase II adjuvant trial of the efficacy, immunogenicity, and safety of GI-4000 plus gem versus gem alone in patients with resected pancreas cancer with activating RAS mutations/survival and immunology analysis of the R1 subgroup. Journal of Clinical Oncology. 2012;30(15_suppl):e14501-e. https://doi.org/10.1200/jco.2012.30.15_suppl.e14501.
Chaft JE, Litvak A, Arcila ME, Patel P, D'Angelo SP, Krug LM, et al. Phase II study of the GI-4000 KRAS vaccine after curative therapy in patients with stage I-III lung adenocarcinoma harboring a KRAS G12C, G12D, or G12V mutation. Clinical lung cancer. 2014;15(6):405–10. https://doi.org/10.1016/j.cllc.2014.06.002.
Ryan MB, Der CJ, Wang-Gillam A, Cox AD. Targeting RAS-mutant cancers: is ERK the key? Trends Cancer. 2015;1(3):183–98. https://doi.org/10.1016/j.trecan.2015.10.001.
Roberts PJ, Der CJ. Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene. 2007;26(22):3291–310. https://doi.org/10.1038/sj.onc.1210422.
Janku F, Yap TA, Meric-Bernstam F. Targeting the PI3K pathway in cancer: are we making headway? Nat Rev Clin Oncol. 2018;15(5):273–91. https://doi.org/10.1038/nrclinonc.2018.28.
Roberts PJ, Der CJ. Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene. 2007;26(22):3291–310. https://doi.org/10.1038/sj.onc.1210422.
Heidorn SJ, Milagre C, Whittaker S, Nourry A, Niculescu-Duvas I, Dhomen N, et al. Kinase-dead BRAF and oncogenic RAS cooperate to drive tumor progression through CRAF. Cell. 2010;140(2):209–21. https://doi.org/10.1016/j.cell.2009.12.040.
Poulikakos PI, Zhang C, Bollag G, Shokat KM, Rosen N. RAF inhibitors transactivate RAF dimers and ERK signalling in cells with wild-type BRAF. Nature. 2010;464(7287):427–30. https://doi.org/10.1038/nature08902.
Peng SB, Henry JR, Kaufman MD, Lu WP, Smith BD, Vogeti S, et al. Inhibition of RAF isoforms and active dimers by LY3009120 leads to anti-tumor activities in RAS or BRAF mutant cancers. Cancer Cell. 2015;28(3):384–98. https://doi.org/10.1016/j.ccell.2015.08.002.
Sullivan RJ, Hollebecque A, Flaherty KT, Shapiro GI, Rodon Ahnert J, Millward MJ, et al. A phase I study of LY3009120, a pan-RAF inhibitor, in patients with advanced or metastatic Cancer. Mol Cancer Ther. 2020;19(2):460–7. https://doi.org/10.1158/1535-7163.mct-19-0681.
Duncan JS, Whittle MC, Nakamura K, Abell AN, Midland AA, Zawistowski JS, et al. Dynamic reprogramming of the Kinome in response to targeted MEK inhibition in triple-negative breast Cancer. Cell. 2012;149(2):307–21. https://doi.org/10.1016/j.cell.2012.02.053.
Ahronian LG, Sennott EM, Van Allen EM, Wagle N, Kwak EL, Faris JE, et al. Clinical acquired resistance to RAF inhibitor combinations in BRAF-mutant colorectal Cancer through MAPK pathway alterations. Cancer Discov. 2015;5(4):358–67. https://doi.org/10.1158/2159-8290.cd-14-1518.
Sullivan RJ, Infante JR, Janku F, Wong DJL, Sosman JA, Keedy V, et al. First-in-class ERK1/2 inhibitor Ulixertinib (BVD-523) in patients with MAPK mutant advanced solid tumors: results of a phase I dose-escalation and expansion study. Cancer Discovery. 2018;8(2):184–95. https://doi.org/10.1158/2159-8290.cd-17-1119.
Tsubaki M, Takeda T, Noguchi M, Jinushi M, Seki S, Morii Y, et al. Overactivation of Akt contributes to MEK inhibitor primary and acquired resistance in colorectal Cancer cells. Cancers (Basel). 2019;11(12):1866. https://doi.org/10.3390/cancers11121866.
Posch C, Moslehi H, Feeney L, Green GA, Ebaee A, Feichtenschlager V, et al. Combined targeting of MEK and PI3K/mTOR effector pathways is necessary to effectively inhibit NRAS mutant melanoma in vitro and in vivo. Proc Natl Acad Sci U S A. 2013;110(10):4015–20. https://doi.org/10.1073/pnas.1216013110.
Engelman JA, Chen L, Tan X, Crosby K, Guimaraes AR, Upadhyay R, et al. Effective use of PI3K and MEK inhibitors to treat mutant Kras G12D and PIK3CA H1047R murine lung cancers. Nat Med. 2008;14(12):1351–6. https://doi.org/10.1038/nm.1890.
Bedard PL, Tabernero J, Janku F, Wainberg ZA, Paz-Ares L, Vansteenkiste J, et al. A phase Ib dose-escalation study of the oral pan-PI3K inhibitor buparlisib (BKM120) in combination with the oral MEK1/2 inhibitor trametinib (GSK1120212) in patients with selected advanced solid tumors. Clinical cancer research : an official journal of the American Association for Cancer Research. 2015;21(4):730–8. https://doi.org/10.1158/1078-0432.ccr-14-1814.
Tolcher AW, Khan K, Ong M, Banerji U, Papadimitrakopoulou V, Gandara DR, et al. Antitumor activity in RAS-driven tumors by blocking AKT and MEK. Clinical cancer research : an official journal of the American Association for Cancer Research. 2015;21(4):739–48. https://doi.org/10.1158/1078-0432.ccr-14-1901.
Yeh JJ, Routh ED, Rubinas T, Peacock J, Martin TD, Shen XJ, et al. KRAS/BRAF mutation status and ERK1/2 activation as biomarkers for MEK1/2 inhibitor therapy in colorectal cancer. Mol Cancer Ther. 2009;8(4):834–43. https://doi.org/10.1158/1535-7163.mct-08-0972.
Jing J, Greshock J, Holbrook JD, Gilmartin A, Zhang X, McNeil E, et al. Comprehensive predictive biomarker analysis for MEK inhibitor GSK1120212. Mol Cancer Ther. 2012;11(3):720–9. https://doi.org/10.1158/1535-7163.mct-11-0505.
Migliardi G, Sassi F, Torti D, Galimi F, Zanella ER, Buscarino M, et al. Inhibition of MEK and PI3K/mTOR suppresses tumor growth but does not cause tumor regression in patient-derived xenografts of RAS-mutant colorectal carcinomas. Clinical cancer research : an official journal of the American Association for Cancer Research. 2012;18(9):2515–25. https://doi.org/10.1158/1078-0432.ccr-11-2683.
Infante JR, Fecher LA, Falchook GS, Nallapareddy S, Gordon MS, Becerra C, et al. Safety, pharmacokinetic, pharmacodynamic, and efficacy data for the oral MEK inhibitor trametinib: a phase 1 dose-escalation trial. Lancet Oncol. 2012;13(8):773–81. https://doi.org/10.1016/s1470-2045(12)70270-x.
Lee MS, Helms TL, Feng N, Gay J, Chang QE, Tian F et al. Efficacy of the combination of MEK and CDK4/6 inhibitors in vitro and in vivo in KRAS mutant colorectal cancer models. Oncotarget. 2016;7(26):39595–608. https://doi.org/10.18632/oncotarget.9153.
Burns MC, Sun Q, Daniels RN, Camper D, Kennedy JP, Phan J, et al. Approach for targeting Ras with small molecules that activate SOS-mediated nucleotide exchange. Proc Natl Acad Sci. 2014;111(9):3401–6. https://doi.org/10.1073/pnas.1315798111.
Hillig RC, Sautier B, Schroeder J, Moosmayer D, Hilpmann A, Stegmann CM, et al. Discovery of potent SOS1 inhibitors that block RAS activation via disruption of the RAS–SOS1 interaction. Proc Natl Acad Sci. 2019;116(7):2551–60. https://doi.org/10.1073/pnas.1812963116.
Gerlach D, Gmachl M, Ramharter J, Teh J, Fu S-C, Trapani F et al. Abstract 1091: BI-3406 and BI 1701963: Potent and selective SOS1::KRAS inhibitors induce regressions in combination with MEK inhibitors or irinotecan. Cancer research. 2020;80(16 Supplement):1091. https://doi.org/10.1158/1538-7445.am2020-1091.
Nichols RJ, Haderk F, Stahlhut C, Schulze CJ, Hemmati G, Wildes D, et al. RAS nucleotide cycling underlies the SHP2 phosphatase dependence of mutant BRAF-, NF1- and RAS-driven cancers. Nat Cell Biol. 2018;20(9):1064–73. https://doi.org/10.1038/s41556-018-0169-1.
Ou SI, Koczywas M, Ulahannan S, Janne P, Pacheco J, Burris H, et al. A12 the SHP2 inhibitor RMC-4630 in patients with KRAS-mutant non-small cell lung Cancer: preliminary evaluation of a first-in-man phase 1 clinical trial. J Thorac Oncol. 2020;15(2):S15–S6. https://doi.org/10.1016/j.jtho.2019.12.041.
Kopetz S, Desai J, Chan E, Hecht JR, O'Dwyer PJ, Lee RJ et al. PLX4032 in metastatic colorectal cancer patients with mutant BRAF tumors. Journal of Clinical Oncology. 2010;28(15_suppl):3534. :https://doi.org/10.1200/jco.2010.28.15_suppl.3534.
Amodio V, Yaeger R, Arcella P, Cancelliere C, Lamba S, Lorenzato A et al. EGFR Blockade Reverts Resistance to KRAS<sup>G12C</sup> Inhibition in Colorectal Cancer. Cancer Discovery. 2020;10(8):1129–39. https://doi.org/10.1158/2159-8290.cd-20-0187. This study demonstrated that RTK dependency and pERK rebound are the cause of limited efficacy of KRASG12C inhibition in colorecal cancer comparing to NSCLC. The improved tumor reduction and sustained pERK inhibition observed in KRASG12C mutated colorecal cancer PDX models treated with AMG510 plus cetuximab highlights that concomitant inhibiton of EGFR and KRASG12C may lead to improved efficacy in patients with KRASG12C mutated colorecal cancer.
Corcoran RB, Ebi H, Turke AB, Coffee EM, Nishino M, Cogdill AP, et al. EGFR-mediated reactivation of MAPK signaling contributes to insensitivity of <em>BRAF</em>−mutant colorectal cancers to RAF inhibition with Vemurafenib. Cancer Discovery. 2012;2(3):227–35. https://doi.org/10.1158/2159-8290.cd-11-0341.
Kopetz S, Grothey A, Yaeger R, Cuyle P-JA, Huijberts S, Schellens JHM et al. Updated results of the BEACON CRC safety lead-in: Encorafenib (ENCO) + binimetinib (BINI) + cetuximab (CETUX) for BRAFV600E-mutant metastatic colorectal cancer (mCRC). Journal of Clinical Oncology. 2019;37(4_suppl):688. https://doi.org/10.1200/JCO.2019.37.4_suppl.688.
Kopetz S, Grothey A, Yaeger R, Van Cutsem E, Desai J, Yoshino T, et al. Encorafenib, Binimetinib, and Cetuximab in BRAF V600E–mutated colorectal Cancer. N Engl J Med. 2019;381(17):1632–43. https://doi.org/10.1056/NEJMoa1908075.
Ryan MB. Fece de la Cruz F, Phat S, Myers DT, Wong E, Shahzade HA et al. vertical pathway inhibition overcomes adaptive feedback resistance to KRAS<sup>G12C</sup> inhibition. Clin Cancer Res. 2020;26(7):1633–43. https://doi.org/10.1158/1078-0432.ccr-19-3523.
Janne PA, Papadopoulous K, Ou SI, Rybkin II and Johnson ML. A Phase 1 clinical trial evaluating the pharmacokinetics (PK), safety, and clinical activity of MRTX849, a mutant-selective small molecule KRAS G12C inhibitor, in advanced solid tumors. Presented at the 2019 AACR-NCI-EORTC International Conference on Molecular Targets and Cancer Therapeutics. October 26–30, 2019; Boston, Massachusetts, Abstract C069.
P. LoRusso, M. Fakih, F.Y.F.L. De Vos, et al. Phase Ib study of ribociclib (R) + trametinib (T) in patients (pts) with metastatic/advanced solid tumours. Annals of Oncology (2020) 31 (suppl_4): S462-S504. https://doi.org/10.1016/annonc/annonc271
Author information
Authors and Affiliations
Contributions
C.W. and M. F. contributed conception design, literature search and review, writing, graphical design, and editing. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
Dr. Fakih reports received Honoraria from Amgen and research funding from Astra Zeneca, Amgen and Novartis. Dr. Fakih reports serving as advisory for Amgen, Array, Bayer and Pfizer and as speaker bureau for Amgen and Guardant Health. Chongkai Wang declared no conflict of interests.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Code Availability
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Evolving Therapies
Rights and permissions
About this article
Cite this article
Wang, C., Fakih, M. Targeting KRAS in Colorectal Cancer. Curr Oncol Rep 23, 28 (2021). https://doi.org/10.1007/s11912-021-01022-0
Accepted:
Published:
DOI: https://doi.org/10.1007/s11912-021-01022-0