Abstract
Purpose of Review
The treatment of advanced melanoma has changed dramatically in recent years with several new drugs having been approved for the treatment of melanoma since 2011. This review aims to evaluate the role of BRAF-targeted therapy for advanced melanoma in the immunotherapy era.
Recent Findings
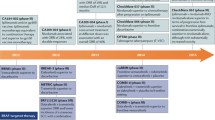
Currently, in patients with BRAF wild-type advanced melanoma, anti-PD-1 (nivolumab or pembrolizumab) is the main treatment. The combination of nivolumab and ipilimumab (anti-CTLA-4) is also an important option for these patients, resulting in a better outcome, but with less favorable toxicity profile. In patients with BRAF mutations, three regimens of BRAF plus MEK inhibitors are now approved (vemurafenib plus cobimetinib, dabrafenib plus trametinib, and encorafenib plus binimetinib), which achieve rapid antitumor responses and a significant survival benefit. In these patients, as well as in BRAF wild-type patients, immunotherapy can be also effective and is regularly used.
Summary
Immunotherapy and targeted therapy have become the new standards of care, substantially improving survival rates. However, many questions still remain unanswered, such as what is the best first- and second-line treatment and the best treatment sequence. New combinations of drugs, targeted therapy combined with immunotherapy, and sequencing approaches are now underway in many ongoing clinical trials.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Korn EL, Liu PY, Lee SJ, Chapman JA, Niedzwiecki D, Suman VJ, et al. Meta-analysis of phase II cooperative group trials in metastatic stage IV melanoma to determine progression-free and overall survival benchmarks for future phase II trials. J Clin Oncol. 2008;26(4):527–34.
Ugurel S, Röhmel J, Ascierto PA, Flaherty KT, Grob JJ, Hauschild A, et al. Survival of patients with advanced metastatic melanoma: the impact of novel therapies-update 2017. Eur J Cancer. 2017;83:247–57.
Topalian SL, Drake CG, Pardoll DM. Targeting the PD-1/B7-H1(PD-L1) pathway to activate anti-tumor immunity. Curr Opin Immunol. 2012;24(2):207–12.
Ascierto PA, Simeone E, Sileni VC, Pigozzo J, Maio M, Altomonte M, et al. Clinical experience with ipilimumab 3 mg/kg: real-world efficacy and safety data from an expanded access programme cohort. J Transl Med. 2014;12:116.
Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–23.
Schadendorf D, Hodi FS, Robert C, Weber JS, Margolin K, Hamid O. Pooled analysis of long-term survival data from phase II and phase III trials of ipilimumab in unresectable or metastatic melanoma. J Clin Oncol. 2015;33(17):1889–94.
Ascierto PA, Del Vecchio M, Robert C, Mackiewicz A, Chiarion-Sileni V, Arance A. Ipilimumab 10 mg/kg versus ipilimumab 3 mg/kg in patients with unresectable or metastatic melanoma: a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol. 2017;18(5):611–22.
Eroglua Z, Kimb DW, Wangc X, Camachod LH, Chmielowskie B, Sejae E, et al. Long term survival with CTLA-4 blockade using tremelimumab. Eur J Cancer. 2015;51(17):2689–97.
Ribas A, Kefford R, Marshall MA, Punt CJ, Haanen JB, Marmol M, et al. Phase III randomized clinical trial comparing tremelimumab with standard-of-care chemotherapy in patients with advanced melanoma. J Clin Oncol. 2013;31(5):616–22.
Topalian SL, Sznol M, McDermott DF, Kluger HM, Carvajal RD, Sharfman WH. Survival, durable tumor remission, and long term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol. 2014;32(10):1020–30.
Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372(4):320–30.
Ascierto PA, Long GV, Robert C, Brady B, Dutriaux C, Di Giacomo AM, et al. Survival outcomes in patients with previously untreated BRAF wild-type advanced melanoma treated with nivolumab therapy: three-year follow-up of a randomized phase 3 trial. JAMA Oncol. 2018;25.
•• Hodi FS, Chiarion-Sileni V, Gonzalez R, Grob JJ, Rutkowski P, Cowey CL, et al. Nivolumab plus ipilimumab or nivolumab alone versus ipilimumab alone in advanced melanoma (CheckMate 067): 4-year outcomes of a multicentre, randomised, phase 3 trial. Lancet Oncol. 2018;19:1480–92 First-line therapy with nivolumab plus ipilimumab or nivolumab alone in patients with advanced melanoma produces a durable, sustained survival benefit.
Ascierto PA, McArthur GA. Checkpoint inhibitors in melanoma and early phase development in solid tumors: what’s the future? J Transl Med. 2017;15(1):173.
Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, et al. 5-Year survival outcomes in patients (pts) with advanced melanoma treated with pembrolizumab (pembro) in KEYNOTE-001. J Clin Oncol. 2018;36(15_suppl):9516–9516.
Ribas A, Puzanov I, Dummer R, Schadendorf D, Hamid O, Robert C. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE- 002): a randomised, controlled, phase 2 trial. Lancet Oncol. 2015;16(8):908–18.
Hamid O, Puzanov I, Dummer R, Schachter J, Daud A, Schadendorf D, et al. Final analysis of a randomised trial comparing pembrolizumab versus investigator-choice chemotherapy for ipilimumab- refractory advanced melanoma. Eur J Cancer. 2017;86:37–45.
• Schachter J, Ribas A, Long GV, Arance A, Grob JJ, Mortier L, et al. Pembrolizumab versus ipilimumab for advanced melanoma: final overall survival results of a multicentre, randomised, open-label phase 3 study (KEYNOTE-006). Lancet. 2017;21;390(10105):1853–62 Pembrolizumab provides superior overall survival versus ipilimumab, with no difference between pembrolizumab dosing schedules.
Puzanov I, Ribas A, Daud A, et al. Pembrolizumab for advanced melanoma: effect of BRAF V600 mutation status and prior BRAF inhibitor therapy. Pigment Cell Melanoma Res J (abstract):392–3.
Simeone E, Grimaldi AM, Festino L, Giannarelli D, Vanella V, Palla M, et al. Correlation between previous treatment with BRAF inhibitors and clinical response to pembrolizumab in patients with advanced melanoma. Oncoimmunology. 2017;6(3):e1283462.
Callahan MK, Kluger H, Postow MA, Segal NH, Lesokhin A, Atkins MB, et al. Nivolumab plus ipilimumab in patients with advanced melanoma: updated survival, response, and safety data in a phase I dose-escalation study. J Clin Oncol. 2018;36(4):391–8.
Postow MA, Chesney J, Pavlick AC, Robert C, Grossmann K, Mc Dermott D, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med. 2015;372(21):2006–17.
Hodi FS, Chesney J, Pavlick AC, Robert C, Grossmann KF, McDermott DF, et al. Combined nivolumab and ipilimumab versus ipilimumab alone in patients with advanced melanoma: 2-year overall survival outcomes in a multicentre, randomised, controlled, phase 2 trial. Lancet Oncol. 2016;17(11):1558–68.
Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373(1):23–34.
Long GV, Atkinson V, Cebon JS, Jameson MB, Fitzharris BM, McNeil CM, et al. Standard-dose pembrolizumab in combination with reduced-dose ipilimumab for patients with advanced melanoma (KEYNOTE029): an open-label, phase 1b trial. Lancet Oncol. 2017;18(9):1202–10.
• Davies MA, Saiag P, Robert C, Grob JJ, Flaherty KT, Arance A, et al. Dabrafenib plus trametinib in patients with BRAFV600-mutant melanoma brain metastases (COMBI-MB): a multicentre, multicohort, open-label, phase 2 trial. Lancet Oncol. 2017;18(7):863–73 In COMBI-MB, 58% of patients responded intracranially to the BRAF inhibitor dabrafenib plus the MEK inhibitor trametinib.
• Tawbi HA, Forsyth PA, Algazi A, Hamid O, Hodi FS, Moschos SJ, et al. Combined nivolumab and ipilimumab in melanoma metastatic to the brain. N Engl J Med. 2018;379(8):722–30 Intracranial responses were achieved by 55% of patients receiving nivolumab plus ipilimumab in CheckMate 204.
•• Long GV, Atkinson V, Lo S, Sandhu S, Guminski AD, Brown MP, et al. Combination nivolumab and ipilimumab or nivolumab alone in melanoma brain metastases: a multicentre randomised phase 2 study. Lancet Oncol. 2018;19(5):672–81 Nivolumab combined with ipilimumab may be considered for upfront therapy in patients with active asymptomatic melanoma brain metastases who have not ha local therapy.
Chang L, Karin M. Mammalian MAP kinase signalling cascades. Nature. 2001;410:37–40.
Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417(6892):949–54.
Chapman PB, Hauschild A, Robert C, Haanen JB, Ascierto P, Larkin J, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364(26):2507–16.
Hauschild A, Grob JJ, Demidov LV, Jouary T, Gutzmer R, Millward M, et al. Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomized controlled trial. Lancet. 2012;380(9839):358–65.
Johannessen CM, Boehm JS, Kim SY, Thomas SR, Wardwell L, Johnson LA, et al. COT drives resistance to RAF inhibition through MAP kinase pathway reactivation. Nature. 2010;468(7326):968–72.
Nazarian R, Shi H, Wang Q, Kong X, Koya RC, Lee H, et al. Melanomas acquire resistance to B-RAF (V600E) inhibition by RTK or N-RAS up regulation. Nature. 2010;468(7326):973–7.
Emery CM, Vijayendran KG, Zipser MC, Sawyer AM, Niu L, Kim JJ, et al. MEK1 mutations confer resistance to MEK and B-RAF inhibition. Proc Natl Acad Sci U S A. 2009;106(48):20411–6.
Poulikakos PI, Persaud Y, Janakiraman M, Kong X, Ng C, Moriceau G, et al. RAF inhibitor resistance is mediated by dimerization of aberrantly spliced BRAF(V600E). Nature. 2011;480(7377):387–90.
Solit DB, Garraway LA, Pratilas CA, Sawai A, Getz G, Basso A, et al. BRAF mutation predicts sensitivity to MEK inhibition. Nature. 2006;439(7074):358–62.
Ribas A, Gonzalez R, Pavlick A, Hamid O, Gajewski TF, Daud A, et al. Combination of vemurafenib and cobimetinib in patients with advanced BRAF(V600)-mutated melanoma: a phase 1b study. Lancet Oncol. 2014;15(9):954–65.
Pavlick A, Ribas A, Gonzalez R, Hamid O, Gajewski T, Daud, et al. Extended follow-up results of phase Ib study (BRIM7) of vemurafenib (VEM) with cobimetinib (COBI) in BRAF-mutant melanoma. J Clin Oncol. 2015;33(Suppl):Abstract 9020.
Daud A, Pavlick AC, Ribas A, Gonzalez R, Lewis KD, Hamid O, et al. Extended follow-up results of a phase 1b study (BRIM7) of cobimetinib (C) combined with vemurafenib (V) in BRAF V600-mutated melanoma. Pigment Cell Mel Res. 2016;31.
Larkin J, Ascierto PA, Dréno B, Atkinson V, Liszkay G, Maio M, et al. Combined vemurafenib and cobimetinib in BRAF-mutated melanoma. N Engl J Med. 2014;371(20):1867–76.
Ascierto PA, McArthur GA, Dréno B, Atkinson V, Liszkay G, Di Giacomo AM, et al. Cobimetinib combined with vemurafenib in advanced BRAF(V600)-mutant melanoma (coBRIM): updated efficacy results from a randomised, double-blind, phase 3 trial. Lancet Oncol. 2016;17(9):1248–60.
• Dreno B, Ascierto PA, McArthur GA, Atkinson V, Liszkay G, Di Giacomo AM, et al. Efficacy and safety of cobimetinib (C) combined with vemurafenib (V) in patients (pts) with BRAFV600 mutation–positive metastatic melanoma: analysis from the 4-year extended follow-up of the phase 3 coBRIM study. J Clin Oncol. 2018;36(15_suppl):9522–9522 Extended follow-up of the phase 3 coBRIM study confirmed the survival benefit of vemurafenib + cobimetinib over vemurafenib alone.
Flaherty KT, Infante JR, Daud A, Gonzalez R, Kefford RF, Sosman J, et al. Combined BRAF and MEK inhibition in melanoma with BRAF V600 mutations. N Engl J Med. 2012 Nov 1;367(18):1694–703.
Daud A, Weber J, Sosman J, Kim K, Gonzalez R, Hamid O, et al. Updated overall survival (OS) results for BRF113220, a phase I–II study of dabrafenib alone versus combined dabrafenib and trametinib in patients with BRAF V600 metastatic melanoma (MM). J Clin Oncol. 2015;33(Suppl):Abstract 9036.
Long GV, Stroyakovskiy D, Gogas H, Levchenko E, de Braud F, Larkin J, et al. Dabrafenib and trametinib versus dabrafenib and placebo for Val600 BRAF-mutant melanoma: a multicentre, double-blind, phase 3 randomized controlled trial. Lancet. 2015;386(9992):444–51.
Long GV, Flaherty KT, Stroyakovskiy D, Gogas H, Levchenko E, de Braud F, et al. Dabrafenib plus trametinib versus dabrafenib monotherapy in patients with metastatic BRAF V600E/K- mutant melanoma: long-term survival and safety analysis of a phase 3 study. Ann Oncol. 2017;28(7):1631–9.
Robert C, Karaszewska B, Schachter, Rutkowski P, Mackiewicz A, Stroyakovskiy D et al. Two year estimate of overall survival in COMBI-v, a randomized, open-label, phase III study comparing the combination of dabrafenib and trametinib versus vemurafenib as first-line therapy in patients with unresectable or metastatic BRAF V600E/K mutation- positive cutaneous melanoma. Presented at European Cancer Congress, Vienna, 25–29 September 2015, abstr 3301.
•• Schadendorf D, Long GV, Stroiakovski D, Karaszewska B, Hauschild A, Levchenko E, et al. Three-year pooled analysis of factors associated with clinical outcomes across dabrafenib and trametinib combination therapy phase 3 randomised trials. Eur J Cancer. 2017;82:45–55 Baseline LDH level and number of organ sites are strongly associated with and/or predictive of PFS and OS in patients with BRAF-mutant melanoma receiving dabrafenib + trametinib.
Sullivan R, Weber J, Patel S, Dummer R, Miller W, Cosgrove D, et al. A phase Ib/II study of BRAF inhibitor (BRAFi) encorafenib (ENCO) plus MEK inhibitor (MEKi) binimetinib (BINI) in cutaneous melanoma patients naive to BRAFi treatment. J Clin Oncol. 2015;33(Suppl):abstr 9007.
• Dummer R, Ascierto PA, Gogas HJ, Arance A, Mandala M, Liszkay G, et al. Encorafenib plus binimetinib versus vemurafenib or encorafenib in patients with BRAF-mutant melanoma (COLUMBUS): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2018;19(10):1315–27 The combination of encorafenib plus binimetinib provided clinically meaningful efficacy with good tolerability by improvements in both progression-free survival and overall survival compared with vemurafenib.
McArthur GA, Chapman PB, Robert C, Larkin J, Haanen JB, Dummer R, et al. Safety and efficacy of vemurafenib in BRAFV600E and BRAFV600K mutation-positive melanoma (BRIM-3): extended follow-up of a phase 3, randomised, open-label study. Lancet Oncol. 2014;15(3):323–32.
Ribas A, Hodi FS, Callahan M, Konto C, Wolchok J. Hepatotoxicity with combination of vemurafenib and ipilimumab. N Engl J Med. 2013 Apr 4;368(14):1365–6.
Hamid O, Patel M, Hodi F, Amaria R, Boasberg P, Sullivan R, et al. Preliminary clinical safety, tolerability and activity of atezolizumab (anti-PDL1) combined with vemurafenib in BRAF V600 metastatic melanoma Pigment. Cell Melanoma Res. 2015.
Minor DR, Puzanov I, Callahan MK, Hug BA, Hoos A. Severe gastrointestinal toxicity with administration of trametinib in combination with dabrafenib and ipilimumab. Pigment Cell Melanoma Res. 2015 Sep;28(5):611–2.
Infante J, Kim TM, Friedmann J et al. Safety and clinical activity of atezolizumab combined with cobimetinib in metastatic melanoma. Presented at the Society for Melanoma Research 2016 Congress, Boston, 6–9 November 2016.
Ribas A, Butler M, Lutzky J, Lawrence D, Robert C, Miller W et al. Phase I study combining anti-PD-L1 (MEDI4736) with BRAF (dabrafenib) and/or MEK (trametinib) inhibitors in advanced melanoma. J Clin Oncol 33(Suppl): Abstract 3003 2015.
Ascierto PA, Ferrucci PF, Stephens R, Del Vecchio M, Atkinson V, Schmidt H, et al. KEYNOTE-022 Part 3: phase 2 randomized study of 1L dabrafenib (D) and trametinib (T) plus pembrolizumab (pembro) or placebo (PBO) for BRAF-mutant advanced melanoma. Ann Oncol. 2018;29(suppl_8).
Dummer R, Schadendorf D, Nathan P, Tawbi H, Robert C, Ascierto PA, et al. The anti-PD-1 antibody spartalizumab (PDR001) in combination with dabrafenib and trametinib in previously untreated patients with advanced BRAF V600-mutant melanoma: first efficacy, safety, and biomarker findings from the part 2 biomarker cohort of COMBi-i. Cancer Res. 2018. https://doi.org/10.1158/1538-7445.
Ascierto PA, Simeone E, Grimaldi AM, Curvietto M, Esposito A, Palmieri G, et al. Do BRAF inhibitors select for populations with different disease progression kinetics? J Transl Med. 2013;11:61.
Ascierto PA, Simeone E, Sileni VC, Del Vecchio M, Marchetti P, Cappellini GC, et al. Sequential treatment with ipilimumab and BRAF inhibitors in patients with metastatic melanoma: data from the Italian cohort of the ipilimumab expanded access program. Cancer Investig. 2014;32(4):144–9.
Daud A, Ribas A, Robert C, Hodi F, Wolchok J, Joshua AM, et al. Long-term efficacy of pembrolizumab (pembro; MK-3475) in a pooled analysis of 655 patients (pts) with advanced melanoma (MEL) enrolled in KEYNOTE-001. J Clin Oncol. 2015;33(Suppl):Abstract 9005.
Weber JS, D'Angelo SP, Minor D, Hodi FS, Gutzmer R, Neyns B, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open- label, phase 3 trial. Lancet Oncol. 2015;16(4):375–84.
Schreuer MS, Chevolet IL, Jansen YJ, Seremet TC, Wilgenhof S. Liénard Det al. Objective responses can be obtained by CTLA-4 inhibition in metastatic melanoma after BRAF inhibitor failure. Melanoma Res. 2015;25(1):68–74.
Ackerman A, Klein O, McDermott DF, Wang W, Ibrahim N, Lawrence DP, et al. Outcomes of patients with metastatic melanoma treated with immunotherapy prior to or after BRAF inhibitors. Cancer. 2014;120(11):1695–701.
Ugurel S, Loquai C, Kähler K, Hassel J, Berking C, Zimmer L, et al. A multicenter DeCOG study on predictors of vemurafenib therapy outcome in melanoma: pretreatment impacts survival. Ann Oncol. 2015;26(3):573–82.
Saab KR, Mooradian MJ, Wang DY, Chon J, Xia CY, Bialczak A, et al. Tolerance and efficacy of BRAF plus MEK inhibition in patients with melanoma who have received PD-1 based therapy. Cancer. 2018;6.
Rozeman E, Sikorska K, Grijpink-Ongering L, Heeres B, Van de Wiel B, Sari A. Phase II study comparing pembrolizumab (PEM) with intermittent/short-term dual MAPK pathway inhibition plus PEM in patients harboring the BRAFV600 mutation (IMPemBra), Annals of Oncology, Volume 29, Issue suppl_8, 1 October 2018.
Young A, Ngiow S, Madore J, Reinhardt J, Landsberg J, Chitsazan A, et al. Targeting adenosine in BRAF-mutant melanoma reduces tumor growth and metastasis. Cancer Res. 2017;77:4684–96. https://doi.org/10.1158/0008-5472.
Martin CA, Cullinane C, Kirby L, Abuhammad S, Lelliott EJ, Waldeck K, et al. Palbociclib synergizes with BRAF and MEK inhibitors in treatment naïve melanoma but not after the development of BRAF inhibitor resistance. Int J Cancer. 2018;142(10):2139–52.
Ascierto PA, Bechter O, Wolter P, Lebbe C, Elez E, Miller W, et al. A phase Ib/II dose-escalation study evaluating triple combination therapy with a BRAF (encorafenib), MEK (binimetinib), and CDK 4/6 (ribociclib) inhibitor in patients (Pts) with BRAF V600-mutant solid tumors and melanoma. Journal of Clinical Oncology. 2017;35(15_suppl):9518–9518.
Funding
This work was supported by grants from Italian Ministry of Health (IT-MOH) through “ Ricerca Corrente.”
AS and MP is funded by Istitutional “Ricerca Corrente.”
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Vito Vanella has received compensation from MSD for service as a consultant.
Lucia Festino has received compensation from MSD for service as a consultant.
Claudia Trojaniello has received compensation from MSD for service as a consultant.
Maria Grazia Vitale declares that she has no conflict of interest.
Antonio Sorrentino declares that he has no conflict of interest.
Miriam Paone declares that she has no conflict of interest.
Paolo A. Ascierto has received research funding from Bristol-Myers Squibb, Roche-Genentech, and Array; has received travel support from MSD; and has received compensation from Bristol-Myers Squibb, Roche-Genentech, MSD, Array BioPharma, Amgen, Novartis, Merck Serono, Pierre Fabre, Incyte, Genmab, NewLink Genetics, MedImmune, AstraZeneca, Syndax, Sun Pharmaceutical Industries Ltd., Sanofi, Idera, Ultimovacs, Sandoz, and Immunocore for service as a consultant.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Melanoma
Rights and permissions
About this article
Cite this article
Vanella, V., Festino, L., Trojaniello, C. et al. The Role of BRAF-Targeted Therapy for Advanced Melanoma in the Immunotherapy Era. Curr Oncol Rep 21, 76 (2019). https://doi.org/10.1007/s11912-019-0827-x
Published:
DOI: https://doi.org/10.1007/s11912-019-0827-x