Abstract
Purpose of Review
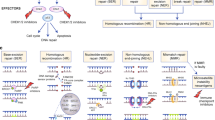
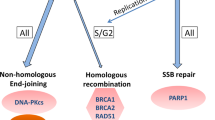
Genomic studies of localized and metastatic prostate cancer have identified a high prevalence of clinically actionable alterations including mutations in DNA repair genes. In this manuscript, we review the current knowledge on DNA repair defects in prostate cancer and provide an overview of how these alterations can be targeted towards a personalized prostate cancer management.
Recent Findings
Twenty to 25% of metastatic prostate cancers harbor defects in DNA repair genes, most commonly in the homologous recombination genes. These defects confer increased sensitivity to platinum chemotherapy or poly (ADP-ribose) polymerase (PARP) inhibitors. Recent trials also support a synergistic effect of combining these therapies with androgen receptor-targeting agents. Identification of mismatch-repair defects could result in defining a prostate cancer population who may benefit from immune checkpoint inhibitors. These data have implications for family testing and early diagnosis, as many of these mutations are linked to inherited risk of prostate cancer.
Summary
The DNA damage repair pathways are clinically relevant in prostate cancer, being a target for precision medicine; combination with standard-of-care androgen receptor (AR)-targeting agents may be synergistic.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Karanika S, Karantanos T, Li L, Corn PG, Thompson TC. DNA damage response and prostate cancer: defects, regulation and therapeutic implications. Oncogene. 2015;34:2815–22.
Ciccia A, Elledge SJ. The DNA damage response: making it safe to play with knives. Mol Cell. Elsevier Inc. 2010;40:179–204. https://doi.org/10.1016/j.molcel.2010.09.019.
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. Elsevier; 2011;144:646–74. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21376230.
Wright WD, Shah SS, Heyer WD. Homologous recombination and the repair of DNA double-strand breaks. J Biol Chem. 2018;293:10524–35.
Li X, Heyer W-D. NIH public access. Cell Res. 2008;18:99–113.
Holloman WK. Unraveling the mechanism of BRCA2 in homologous recombination. Nat Struct Mol Biol Nature Publishing Group. 2011;18:748–54. https://doi.org/10.1038/nsmb.2096.
Castroviejo-Bermejo M, Cruz C, Llop-Guevara A, Gutiérrez-Enríquez S, Ducy M, Ibrahim YH, et al.. EMBO Mol Med. 2018;e9172. https://doi.org/10.15252/emmm.201809172
Brown JS, O’Carrigan B, Jackson SP, Yap TA. Targeting DNA repair in cancer: beyond PARP inhibitors. Cancer Discov. 2017;7:20–37.
Daley JM, Sung P. 53BP1, BRCA1, and the choice between recombination and end joining at DNA double-strand breaks. Mol Cell Biol. 2014;34:1380–8. https://doi.org/10.1128/MCB.01639-13.
Schiewer MJ, Knudsen KE. Cold Spring Harb Perspect Med. 2018;a030486. https://doi.org/10.1101/cshperspect.a030486
Liu D, Keijzers G, Rasmussen LJ. DNA mismatch repair and its many roles in eukaryotic cells. Mutat Res Rev Mutat Res. Elsevier B.V. 2017;773:174–87. https://doi.org/10.1016/j.mrrev.2017.07.001.
Farmer H, McCabe N, Lord CJ, Tutt ANJ, Johnson DA, Richardson TB, et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature. Macmillan Magazines Ltd. 2005;434:917. https://doi.org/10.1038/nature03445.
Bryant HE, Schultz N, Thomas HD, Parker KM, Flower D, Lopez E, et al. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature. 2005;434:913–7.
Fong PC, Boss DS, Yap TA, Tutt A, Wu P, Mergui-Roelvink M, et al. Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N Engl J Med. Massachusetts Medical Society. 2009;361:123–34. https://doi.org/10.1056/NEJMoa0900212.
Tutt A, Robson M, Garber JE, Domchek SM, Audeh MW, Weitzel JN, et al. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and advanced breast cancer: a proof-of-concept trial. Lancet (London, England). Elsevier; 2010 [cited 2018 Dec 27];376:235–44. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20609467.
Ledermann J, Harter P, Gourley C, Friedlander M, Vergote I, Rustin G, et al. Olaparib maintenance therapy in platinum-sensitive relapsed ovarian cancer. N Engl J Med. Massachusetts Medical Society. 2012;366:1382–92. https://doi.org/10.1056/NEJMoa1105535.
Swisher EM, Lin KK, Oza AM, Scott CL, Giordano H, Sun J, et al. Rucaparib in relapsed, platinum-sensitive high-grade ovarian carcinoma (ARIEL2 Part 1): an international, multicentre, open-label, phase 2 trial. Lancet Oncol. Elsevier; 2017 [cited 2018 Dec 27];18:75–87. Available from: http://www.ncbi.nlm.nih.gov/pubmed/27908594.
Robson M, Im S-A, Senkus E, Xu B, Domchek SM, Masuda N, et al. Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. N Engl J Med. Massachusetts Medical Society. 2017;377:523–33. https://doi.org/10.1056/NEJMoa1706450.
Moore K, Colombo N, Scambia G, Kim B-G, Oaknin A, Friedlander M, et al. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. Massachusetts Medical Society. 2018;379:2495–505. https://doi.org/10.1056/NEJMoa1810858.
Mirza MR, Monk BJ, Herrstedt J, Oza AM, Mahner S, Redondo A, et al. Niraparib maintenance therapy in platinum-sensitive, recurrent ovarian cancer. N Engl J Med. Massachusetts Medical Society. 2016;375:2154–64. https://doi.org/10.1056/NEJMoa1611310.
Gubin MM, Zhang X, Schuster H, Caron E, Ward JP, Noguchi T, et al. Checkpoint blockade cancer immunotherapy targets tumour-specific mutant antigens. Nature. Nature Publishing Group, a division of Macmillan Publishers Limited. All Rights Reserved. 2014;515:577. https://doi.org/10.1038/nature13988.
Van Allen EM, Miao D, Schilling B, Shukla SA, Blank C, Zimmer L, et al. Genomic correlates of response to CTLA-4 blockade in metastatic melanoma. Science (80- ). 2015;350:207 LP-211 Available from: http://science.sciencemag.org/content/350/6257/207.abstract.
Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. Massachusetts Medical Society. 2015;372:2509–20. https://doi.org/10.1056/NEJMoa1500596.
Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK, et al. Mismatch-repair deficiency predicts response of solid tumors to PD-1 blockade. Science (80- ). 2017;eaan6733. Available from: http://science.sciencemag.org/content/early/2017/06/07/science.aan6733.abstract
Kote-Jarai Z, Leongamornlert D, Saunders E, Tymrakiewicz M, Castro E, Mahmud N, et al. BRCA2 is a moderate penetrance gene contributing to young-onset prostate cancer: implications for genetic testing in prostate cancer patients. Br J Cancer. The Author(s). 2011;105:1230. https://doi.org/10.1038/bjc.2011.383.
Leongamornlert D, Saunders E, Dadaev T, Tymrakiewicz M, Goh C, Jugurnauth-Little S, et al. Frequent germline deleterious mutations in DNA repair genes in familial prostate cancer cases are associated with advanced disease. Br J Cancer. The Author(s). 2014;110:1663. https://doi.org/10.1038/bjc.2014.30.
Edwards SM, Kote-Jarai Z, Meitz J, Hamoudi R, Hope Q, Osin P, et al. Two percent of men with early-onset prostate cancer harbor germline mutations in the BRCA2 gene. Am J Hum Genet. 2003;72:1–12 Available from: http://linkinghub.elsevier.com/retrieve/pii/S0002929707604996.
Castro E, Goh C, Olmos D, Saunders E, Leongamornlert D, Tymrakiewicz M, et al. Germline BRCA mutations are associated with higher risk of nodal involvement, distant metastasis, and poor survival outcomes in prostate cancer. J Clin Oncol. 2013;31:1748–57.
Castro E, Goh C, Leongamornlert D, Saunders E, Tymrakiewicz M, Dadaev T, et al. Effect of BRCA mutations on metastatic relapse and cause-specific survival after radical treatment for localised prostate cancer. Eur Urol. Elsevier. 2015;68:186–93. https://doi.org/10.1016/j.eururo.2014.10.022.
Carter HB, Helfand B, Mamawala M, Wu Y, Landis P, Yu H, et al. Germline mutations in ATM and BRCA1/2 are associated with grade reclassification in men on active surveillance for prostate cancer. Eur Urol. Elsevier. 2019. https://doi.org/10.1016/j.eururo.2018.09.021.
Beltran H, Yelensky R, Frampton GM, Park K, Downing SR, MacDonald TY, et al. Targeted next-generation sequencing of advanced prostate cancer identifies potential therapeutic targets and disease heterogeneity. Eur Urol. Elsevier. 2013;63:920–6. https://doi.org/10.1016/j.eururo.2012.08.053.
Grasso CS, Wu YM, Robinson DR, Cao X, Dhanasekaran SM, Khan AP, et al. The mutational landscape of lethal castration-resistant prostate cancer. Nature. 2012;487:239–43.
Zehir A, Benayed R, Shah RH, Syed A, Middha S, Kim HR, et al. Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10,000 patients. Nat Med. Nature Publishing Group, a division of Macmillan Publishers Limited. All Rights Reserved. 2017;23:703. https://doi.org/10.1038/nm.4333.
• Abeshouse A, Ahn J, Akbani R, Ally A, Amin S, Andry CD, et al. The molecular taxonomy of primary prostate cancer. Cell. 2015;163:1011–25 This study is a comprehensive molecular analysis of primary prostate carcinomas from the TCGA consortium that shows the frequent inactivation of DNA repair genes.
• Robinson D, Van Allen EM, Wu YM, Schultz N, Lonigro RJ, Mosquera JM, et al. Integrative clinical genomics of advanced prostate cancer. Cell. 2015;161:1215–28 Comprehensive profiling of somatic genomic alterations in a metastatic tumor cohort (mCRPC). Aberrations of key DDR genes (BRCA2, BRCA1, and ATM were observed at higher frequencies compared to primary prostate cancer.
Wu YM, Cieślik M, Lonigro RJ, Vats P, Reimers MA, Cao X, et al. Inactivation of CDK12 delineates a distinct immunogenic class of advanced prostate cancer. Cell. 2018;173:1770–1782.e14.
Shelley MD, Mason MD. Metastatic prostate cancer. Evidence-Based Urol. 2010:293–303.
•• Pritchard CC, Mateo J, Walsh MF, De Sarkar N, Abida W, Beltran H, et al. Inherited DNA-repair gene mutations in men with metastatic prostate cancer. N Engl J Med. 2016;375:443–53 This study assesses the presence of germline mutations in DDR genes. Showing a higher overall frequency (11.8%) in metastatic prostate cancer.
Pritchard CC, Morrissey C, Kumar A, Zhang X, Smith C, Coleman I, et al. Complex MSH2 and MSH6 mutations in hypermutated microsatellite unstable advanced prostate cancer. Nat Commun. Nature Publishing Group. 2014;5:1–6. https://doi.org/10.1038/ncomms5988.
Antonarakis ES, Shaukat F, Isaacsson Velho P, Kaur H, Shenderov E, Pardoll DM, et al. Clinical features and therapeutic outcomes in men with advanced prostate cancer and DNA mismatch repair gene mutations. Eur Urol. European Association of Urology. 2018:1–5. https://doi.org/10.1016/j.eururo.2018.10.009.
Komura K, Yoshikawa Y, Shimamura T, Chakraborty G, Gerke TA, Hinohara K, et al. ATR inhibition controls aggressive prostate tumors deficient in Y-linked histone demethylase KDM5D. J Clin Invest. 2018;128:2979–95.
•• Armenia J, Wankowicz SAM, Liu D, Gao J, Kundra R, Reznik E, et al. The long tail of oncogenic drivers in prostate cancer. Nat Genet. 2018;50:645–51. https://doi.org/10.1038/s41588-018-0078-z Collection and reanalysis of exome sequencing data from multiple studies that enabled the comparison between primary and metastatic tumors. Enrichment of alterations in DDR genes was observed in metastatic samples.
Abida W, Armenia J, Gopalan A, Brennan R, Walsh M, Barron D, et al. Prospective genomic profiling of prostate cancer across disease states reveals germline and somatic alterations that may affect clinical decision making. JCO Precis Oncol. American Society of Clinical Oncology. 2017;1:1–16. https://doi.org/10.1200/PO.17.00029.
Na R, Zheng SL, Han M, Yu H, Jiang D, Shah S, et al. Germline mutations in ATM and BRCA1/2 distinguish risk for lethal and indolent prostate cancer and are associated with early age at death. Eur Urol. Elsevier. 2017;71:740–7. https://doi.org/10.1016/j.eururo.2016.11.033.
National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Prostate Cancer. Version 4.2018. www.nccn.org. Accessed 15 Dec 2018.
Annala M, Struss WJ, Warner EW, Beja K, Vandekerkhove G, Wong A, et al. Treatment outcomes and tumor loss of heterozygosity in germline DNA repair-deficient prostate cancer. Eur Urol. Elsevier. 2017;72:34–42. https://doi.org/10.1016/j.eururo.2017.02.023.
Annala M, Vandekerkhove G, Khalaf D, Taavitsainen S, Beja K, Warner EW, et al. Circulating tumor DNA genomics correlate with resistance to abiraterone and enzalutamide in prostate cancer. Cancer Discov. 2018;8:444–57.
Mateo J, Cheng HH, Beltran H, Dolling D, Xu W, Pritchard CC, et al. Clinical outcome of prostate cancer patients with germline DNA repair mutations: retrospective analysis from an international study. Eur Urol. Elsevier. 2018;73:687–93. https://doi.org/10.1016/j.eururo.2018.01.010.
Antonarakis ES, Lu C, Luber B, Liang C, Wang H, Chen Y, et al. Germline DNA-repair gene mutations and outcomes in men with metastatic castration-resistant prostate cancer receiving first-line abiraterone and enzalutamide. Eur Urol. Elsevier. 2018;74:218–25. https://doi.org/10.1016/j.eururo.2018.01.035.
Hussain M, Tangen CM, Thompson IM, Swanson GP, Wood DP, Sakr W, et al. Phase III Intergroup Trial of Adjuvant Androgen Deprivation With or Without Mitoxantrone Plus Prednisone in Patients With High-Risk Prostate Cancer After Radical Prostatectomy: SWOG S9921. J Clin Oncol. American Society of Clinical Oncology. 2018;36:1498–504. https://doi.org/10.1200/JCO.2017.76.4126.
Castro E, Romero-Laorden N, del Pozo A, Lozano R, Medina A, Puente J, et al. PROREPAIR-B: A Prospective Cohort Study of the Impact of Germline DNA Repair Mutations on the Outcomes of Patients With Metastatic Castration-Resistant Prostate Cancer. J Clin Oncol. American Society of Clinical Oncology; 2019;JCO.18.00358. https://doi.org/10.1200/JCO.18.00358.
de Bono J, Ramanathan RK, Mina L, Chugh R, Glaspy J, Rafii S, et al. Phase I, dose-escalation, 2-part trial of poly(ADP-ribose) polymerase inhibitor talazoparib in patients with advanced germline BRCA1/2 mutations and selected sporadic cancers. Cancer Discov. 2017;CD-16-1250. Available from: http://cancerdiscovery.aacrjournals.org/content/early/2017/02/25/2159-8290.CD-16-1250.abstract
• Sandhu SK, Schelman WR, Wilding G, Moreno V, Baird RD, Miranda S, et al. The poly(ADP-ribose) polymerase inhibitor niraparib (MK4827) in BRCA mutation carriers and patients with sporadic cancer: a phase 1 dose-escalation trial. Lancet Oncol. Elsevier; 2013 [cited 2018 Dec 27];14:882–92. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23810788. Niraparib phase I including a prostate cancer expansion cohort.
Kaufman B, Shapira-Frommer R, Schmutzler RK, Audeh MW, Friedlander M, Balmaña J, et al. Olaparib monotherapy in patients with advanced cancer and a germline BRCA1/2 mutation. J Clin Oncol. American Society of Clinical Oncology. 2014;33:244–50. https://doi.org/10.1200/JCO.2014.56.2728.
Ang JE, Gourley C, Powell CB, High H, Shapira-Frommer R, Castonguay V, et al. Efficacy of chemotherapy in BRCA1/2 mutation carrier ovarian cancer in the setting of PARP inhibitor resistance: a multi-institutional study. Clin Cancer Res. 2013;19:5485–93.
Goodall J, Mateo J, Yuan W, Mossop H, Porta N, Miranda S, et al. Circulating cell-free DNA to guide prostate cancer treatment with PARP inhibition. Cancer Discov. 2017;7:1006–17.
• Abida W, Bryce AH, Balar AV, Chatta GS, Dawson NA, Guancial EA, et al. TRITON2: An international, multicenter, open-label, phase II study of the PARP inhibitor rucaparib in patients with metastatic castration-resistant prostate cancer (mCRPC) associated with homologous recombination deficiency (HRD). J Clin Oncol . American Society of Clinical Oncology; 2018;36:TPS388-TPS388. Available from: https://doi.org/10.1200/JCO.2018.36.6_suppl.TPS388 Preliminary results of a phase II trial of rucaparib in patients with mCRPC; HR alterations deteced by NGS are presented.
Sternberg CN, Davis ID, Mardiak J, Szczylik C, Lee E, Wagstaff J, et al. Pazopanib in Locally Advanced or Metastatic Renal Cell Carcinoma: Results of a Randomized Phase III Trial. J Clin Oncol. American Society of Clinical Oncology. 2010;28:1061–8. https://doi.org/10.1200/JCO.2009.23.9764.
Aparicio AM, Harzstark AL, Corn PG, Wen S, Araujo JC, Tu S-M, et al. Platinum-based chemotherapy for variant castrate-resistant prostate cancer. Clin Cancer Res. 2013;19:3621 LP-3630 Available from: http://clincancerres.aacrjournals.org/content/19/13/3621.abstract.
Cheng HH, Pritchard CC, Boyd T, Nelson PS, Montgomery B. Biallelic inactivation of BRCA2</em> in platinum-sensitive metastatic castration-resistant prostate cancer. Eur Urol . Elsevier. 2016;69:992–5. https://doi.org/10.1016/j.eururo.2015.11.022.
Zafeiriou Z, Bianchini D, Chandler R, Rescigno P, Yuan W, Carreira S, et al. Genomic analysis of three metastatic prostate cancer patients with exceptional responses to carboplatin indicating different types of DNA repair deficiency. Eur Urol. Elsevier. 2019;75:184–92. https://doi.org/10.1016/j.eururo.2018.09.048.
Kumar A, Coleman I, Morrissey C, Zhang X, True LD, Gulati R, et al. Substantial interindividual and limited intraindividual genomic diversity among tumors from men with metastatic prostate cancer. Nat Med. Nature Publishing Group, a division of Macmillan Publishers Limited. All Rights Reserved. 2016;22:369. https://doi.org/10.1038/nm.4053.
Abida W, ML C, Armenia J, et al. Analysis of the prevalence of microsatellite instability in prostate cancer and response to immune checkpoint blockade . JAMA Oncol. 2018; https://doi.org/10.1001/jamaoncol.2018.5801
Quigley DA, Dang HX, Zhao SG, Lloyd P, Aggarwal R, Alumkal JJ, et al. Genomic hallmarks and structural variation in metastatic prostate cancer. Cell. 2018;174:758–769.e9.
Hansen AR, Massard C, Ott PA, Haas NB, Lopez JS, Ejadi S, et al. Pembrolizumab for advanced prostate adenocarcinoma: findings of the KEYNOTE-028 study. Ann Oncol. 2018;29:1807–13. https://doi.org/10.1093/annonc/mdy232.
Graff JN, Alumkal JJ, Drake CG, Thomas GV, Redmond WL, Farhad M, et al. Early evidence of anti-PD-1 activity in enzalutamide-resistant prostate cancer. Oncotarget. 2016;7:52810–7 Available from: http://www.oncotarget.com/fulltext/10547.
De Bono JS, Goh JCH, Ojamaa K, Piulats Rodriguez JM, Drake CG, Hoimes CJ, et al. KEYNOTE-199: pembrolizumab (pembro) for docetaxel-refractory metastatic castration-resistant prostate cancer (mCRPC). J Clin Oncol. American Society of Clinical Oncology. 2018;36:5007. https://doi.org/10.1200/JCO.2018.36.15_suppl.5007.
Yin Y, Li R, Xu K, Ding S, Li J, Baek GH, et al. Androgen receptor variants mediate DNA repair after prostate cancer irradiation. Cancer Res. 2017;77:4745–54.
Goodwin JF, Schiewer MJ, Dean JL, Schrecengost RS, de Leeuw R, Han S, et al. A hormone-DNA repair circuit governs the response to genotoxic insult. Cancer Discov. 2013;3:1254–71.
Polkinghorn WR, Parker JS, Lee MX, Kass EM, Spratt DE, Iaquinta PJ, et al. Androgen receptor signaling regulates DNA repair in prostate cancers. Cancer Discov. 2013;3:1245–53.
Li L, Karanika S, Yang G, Wang J, Park S, Broom BM, et al. Androgen receptor inhibitor-induced “BRCAness” and PARP inhibition are synthetically lethal for castration-resistant prostate cancer. Sci Signal. 2017;10:eaam7479.
Asim M, Tarish F, Zecchini HI, Sanjiv K, Gelali E, Massie CE, et al. Synthetic lethality between androgen receptor signalling and the PARP pathway in prostate cancer. Nat Commun. Springer US; 2017;8. https://doi.org/10.1038/s41467-017-00393-y
Schiewer MJ, Goodwin JF, Han S, Chad Brenner J, Augello MA, Dean JL, et al. Dual roles of PARP-1 promote cancer growth and progression. Cancer Discov. 2012;2:1134–49.
Schiewer MJ, Mandigo AC, Gordon N, Huang F, Gaur S, de Leeuw R, et al. PARP-1 regulates DNA repair factor availability. EMBO Mol Med. 2018;e8816. https://doi.org/10.15252/emmm.201708816
Brenner JC, Ateeq B, Li Y, Yocum AK, Cao Q, Asangani IA, et al. Mechanistic rationale for inhibition of poly(ADP-ribose) polymerase in ETS gene fusion-positive prostate cancer. Cancer Cell. Elsevier; 2011 [cited 2018 Dec 27];19:664–78. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21575865.
•• Mateo J, Carreira S, Sandhu S, Miranda S, Mossop H, Perez-Lopez R, et al. DNA-repair defects and olaparib in metastatic prostate cancer. N Engl J Med. 2015;373:1697–708. https://doi.org/10.1056/NEJMoa1506859 This study presents the results of the TOPARP-A clinical trial (proof of concept phase II study of olaparib in mCRPC), showing that patients no longer responding to standard treatments and harboring defects in DDR genes had a high response rate when treated with PARP inhibitors (olaparib).
• Clarke N, Wiechno P, Alekseev B, Sala N, Jones R, Kocak I, et al. Olaparib combined with abiraterone in patients with metastatic castration-resistant prostate cancer: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol. Elsevier; 2018 [cited 2018 Dec 28];19:975–86. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29880291. Results of a randomized phase II of combinations therapies (abiraterone and olaparib).
Fizazi K, Tran N, Fein L, Matsubara N, Rodriguez-Antolin A, Alekseev BY, et al. Abiraterone plus prednisone in metastatic, castration-sensitive prostate cancer. N Engl J Med. Massachusetts Medical Society. 2017;377:352–60. https://doi.org/10.1056/NEJMoa1704174.
Hussain M, Fizazi K, Saad F, Rathenborg P, Shore N, Ferreira U, et al. Enzalutamide in men with nonmetastatic, castration-resistant prostate cancer. N Engl J Med. Massachusetts Medical Society. 2018;378:2465–74. https://doi.org/10.1056/NEJMoa1800536.
Alexandrov LB, Nik-Zainal S, Wedge DC, Aparicio SAJR, Behjati S, Biankin AV, et al. Signatures of mutational processes in human cancer. Nature. Nature Publishing Group, a division of Macmillan Publishers Limited. All Rights Reserved. 2013;500:415. https://doi.org/10.1038/nature12477.
Macintyre G, Goranova TE, De Silva D, Ennis D, Piskorz AM, Eldridge M, et al. Copy number signatures and mutational processes in ovarian carcinoma. Nat Genet. 2018;50:1262–70. https://doi.org/10.1038/s41588-018-0179-8.
Cruz C, Castroviejo-Bermejo M, Gutiérrez-Enríquez S, Llop-Guevara A, Ibrahim YH, Gris-Oliver A, et al. RAD51 foci as a functional biomarker of homologous recombination repair and PARP inhibitor resistance in germline BRCA-mutated breast cancer. Ann Oncol. 2018;29:1203–10. https://doi.org/10.1093/annonc/mdy099.
Funding
J. Mateo is supported by a Prostate Cancer Foundation and has received research funding from Fundació Obra Social La Caixa, Cellex Foundation, FERO, Sociedad Española de Oncología Médica (SEOM) and the US Department of Defense.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Alejandro Athie declares that he has no conflict of interest.
Sara Arce-Gallego declares that she has no conflict of interest.
Macarena Gonzalez has received compensation from Roche for service as a consultant/advisor and has received reimbursement for travel/accommodation expenses from Astellas, Bayer, and Lilly.
Rafael Morales-Barrera has received compensation from Sanofi Aventis, Bayer, Janssen, AstraZeneca, Merck Sharp & Dohme, and Asofarma for service as a consultant/advisor and has received reimbursement for travel/accommodation expenses from Roche, Sanofi Aventis, Astellas, Janssen, Merck Sharp & Dohme, Bayer, Pharmacyclics, Clovis Oncology, and Lilly.
Cristina Suarez has received compensation from Roche for service as a consultant, compensation from Bristol-Myers Squibb, Pfizer, and Ipsen for service on advisory boards and on speaker’s bureaus, and has received reimbursement for travel/accommodation expenses from Bristol-Myers Squibb, Pfizer, and Roche.
Teresa Casals Galobart declares that she has no conflict of interest.
Gonzalo Hernandez Viedma declares that he has no conflict of interest.
Joan Carles has received compensation from Bayer, Johnson & Johnson, Bristol-Myers Squibb, Astellas, Pfizer, Sanofi, MSD Oncology, Roche, and AstraZeneca for service on advisory boards and has received compensation from Bayer, Johnson & Johnson, Asofarma, and Astellas for service on speaker’s bureaus.
Joaquin Mateo has received compensation from AstraZeneca, Roche, and Janssen for service on advisory boards, compensation from Sanofi and Astellas for service on speaker’s bureaus, and has received reimbursement for travel/accommodation expenses from AstraZeneca.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Genitourinary Cancers
Rights and permissions
About this article
Cite this article
Athie, A., Arce-Gallego, S., Gonzalez, M. et al. Targeting DNA Repair Defects for Precision Medicine in Prostate Cancer. Curr Oncol Rep 21, 42 (2019). https://doi.org/10.1007/s11912-019-0790-6
Published:
DOI: https://doi.org/10.1007/s11912-019-0790-6