Abstract
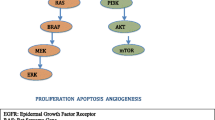
The epidermal growth factor receptor (EGFR) is a promising target in the treatment of advanced stage non-small-cell lung cancer (NSCLC). Currently erlotinib and gefitinib are approved by the US Food and Drug Administration, whereas cetuximab is being studied for use in NSCLC. Erlotinib has shown a survival advantage in patients with advanced NSCLC. Further studies have identified female sex, nonsmokers, Asian race, good performance status, and adenocarcinoma histology as predictors of patient response to these agents. A genetic mutation in EGFR has also been correlated with an increase in response.
Similar content being viewed by others
References
Jemel A, Murray T, Ward E, et al.: Cancer statistics, 2005. CA Cancer J Clin 2005, 55:10–30.
Byrne B, Crawford J, Gay G, et al.: Trends in chemotherapy use and survival for patients with metastatic non-small cell lung cancer [abstract]. Proc ASCO 2004, 13:7082.
Cella D, Bonomi A, Lloyd S, et al.: Reliability and validity of the Functional Assessment of Cancer Therapy—Lung (FACT-L) quality of life instrument. Lung Cancer 1995, 12:199–220.
Schiller J, Harrington D, Belani C, et al.: Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med 2002, 346:92–98.
Shepherd F, Dancey J, Ramlau R, et al.: Prospective randomized trial of docetaxel versus best supportive care in patients with non-small-cell lung cancer previously treated with platinumbased chemotherapy. J Clin Oncol 2000, 18:2095–2103.
Massarelli F, Andre F, Liu D, et al.: A retrospective analysis of the outcome of patients who have received two prior chemotherapy regimens including platinum and docetaxel for recurrent non-small-cell lung cancer. Lung Cancer 2002, 39:55–61.
Arteaga CL: ErbB-targeted therapeutic approaches in human cancer. Exp Cell Res 2003, 284:122–130.
El-Rayes B, LoRusso P: Targeting the epidermal growth factor receptor. Br J Cancer 2004, 91:418–424.
Perez-Soler R: HER1/EGFR targeting: refining the strategy. Oncologist 2004, 9:58–67.
Kris M, Natale R, Herbst R: Efficacy of gefitinib, an inhibitor of the epidermal growth factor receptor tyrosine kinase, in symptomatic patients with non-small cell lung cancer. JAMA 2003, 16:2149–2158. This report describes the IDEAL-2 study, which led to FDA approval of gefitinib. It showed a response rate in a heavily pretreated population of patients with NSCLC.
Fukuoka M, Yano S, Giaccone G, et al.: Multi-institutional randomized phase II trial of gefitinib for previously treated patients with advanced non-small-cell lung cancer. J Clin Oncol 2003, 21:2237–2246. IDEAL-1 study led to the gefitinib dose of 250 mg a day and suggested a response to gefitinib as a second- or third-line agent
Cohen M, Williams G, Sridhara R, et al.: FDA drug approval summary: gefitinib (ZD1839) (Iressa) tablets. Oncologist 2003, 8:303–306.
AstraZeneca: Physician letter. http://www.irressa.us.com/ prof.usp Accessed December 17, 2004.
Shepherd F, Pereira J, Ciuleanu T, et al.: A randomized placebocontrolled of erlotinib in patients with advanced non-small cell lung cancer following failure of 1st line or 2nd line chemotherapy. A National Cancer Institute of Canada Clinical Trials Group [abstract]. J Clin Oncol 2004, 22:7022.
Ochs J, Grous K, Warner L: Final survival and safety results for 21,064 non-small-cell lung cancer (NSCLC) patients who received compassionate use gefitinib in a U.S. expanded access program (EAP) [abstract]. J Clin Oncol 2004, 22:7060.
Miller V, Kris M, Shas N, et al.: Bronchioalveolar pathologic subtype and smoking history predict sensitivity to gefitinib in advanced non-small-cell lung cancer. J Clin Oncol 2004, 22:1103–1109.
Kris M, Sandler A, Miller V, et al.: Cigarette smoking history predicts sensitivity to erlotinib: results of a phase II trial in patients with bronchioalveolar carcinoma (BAC) [abstract]. J Clin Oncol 2004, 22:7062.
Janne P, Gurubhagavatula S, Yeap B, et al.: Outcomes of patients with advanced non-small cell lung cancer treated with gefitinib (ZD1839,’Iressa’) on an expanded access study. Lung Cancer 2004, 44:221–230.
Kaneda H, Tamura K, Kurata T, et al.: Retrospective analysis of the predictive factors associated with the response and survival benefit of gefitinib in patients with advanced nonsmall-cell lung cancer. Lung Cancer 2004, 46:247–254.
Kim Y, Goto K, Ishii GL: Association of the papillary subtype of lung adenocarcinoma with response to gefitinib [abstract]. Proc ASCO 2004, 23:7092.
Ciardiello F, Caputo R, Bianco R, et al.: Antitumor effect and potentiation of cytotoxic drugs activity in human cancer cells by ZD-1839 (Iressa), an epidermal growth factor receptorselective tyrosine kinase inhibitor. Clin Cancer Res 2000, 6:4885–4892.
Sirotnak FM, Zakaowski MF, Miller VA, et al.: Efficacy of cytotoxic agents against human tumor xenografts is markedly enhanced by coadministration of ZD1839 (Iressa), an inhibitor of EGFR tyrosine kinase. Clin Cancer Res 2000, 6:4885–4892.
Miller VA, Johnson DH, Krug LM, et al.: Pilot trial of epidermal growth factor receptor tyrosine kinase inhibitor gefitinib plus carboplatin and paclitaxel in patients with stage IIIB or IV non-small-cell lung cancer. J Clin Oncol 2003, 21:2094–2100.
Giaccone G, Herbst R, Manegold G, et al.: Gefitinib in combination with gemcitabine and cisplatin in advanced non-smallcell lung cancer: a phase III trial—INTACT 1. J Clin Oncol 2004, 22:777–784. This study failed to show a benefit for addition of gefitinib to firstline therapy in NSCLC. Its twin study is by Herbst et al. [25].
Herbst R, Giaccone G, Schiller J, et al.: Gefitinib in combination with paclitaxel and carboplatin in advanced non-smallcell lung cancer: a phase III trial—INTACT 2. J Clin Oncol 2004, 22:785–794.
Herbst R, Prager D, Hermann R, et al.: TRIBUTE-A phase III trial of erlotinib HCL OSI-774) combined with carboplatin and paclitaxel (CP) chemotherapy in advanced non-small cell lung cancer (NSCLC) [abstract]. Proc ASCO 2004, 23:7012.
Gatzemeier U, Pluzanska A, Szczesna A, et al.: Results of a phase III trial of erlotinib (OSI-744) combined with cisplatin and gemcitabine (GC) chemotherapy in advanced non-small cell lung cancer (NSCLC) [abstract]. Proc ASCO 2004, 23:7010.
Miller V, Herbst R, Prager D, et al.: Long survival of never smoking non-small cell lung cancer (NSCLC) patients (pt) treated with erlotinib HCL (OSI-774) and chemotherapy: Sub-group analysis of TRIBUTE [abstract]. Proc ASCO 2004, 23:7061.
Lynch T, Bell D, Sordella R, et al.: Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med 2004, 350:2129–2139. In this landmark study researchers discovered a mutation in EGFR that led to increased response to gefitinib.
Paez J, Janne P, Lee J, et al.: EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science 2004, 304:1497–1500. This study showed an increased rate of mutations in EGFR in subpopulations that were most likely to respond to gefitinib.
Pao W, Miller V, Zakowski M, et al.: EGF receptor gene mutations are common in lung cancers from ‘never smokers’ and are associated with sensitivity of tumors to gefitinib and erlotinib. PNAS 2004, 101:13306–13311.
Camus P, Kudoh S, Ebina M: Interstitial lung disease associated with drug therapy. Br J Cancer 2004, 91:S18-S23.
Seto T, Yamamoto N: Interstitial lung disease (ILD) induced by gefitinib in patients with advanced non-small cell lung cancer (NSCLC): results of a West Japan Thoracic Oncology group (WJTOG) epidemiological survey [abstract]. Proc ASCO 2004, 23:7064.
Hotta K, Harita S, Bessho A, et al.: Interstitial lung disease (ILD) during gefitinib treatment in Japanese patients with non-small cell lung cancer (NSCLC): Okyama Lung Cancer Study Group [abstract]. Proc ASCO 2004, 23:7063.
Cappuzzo F, Bartolini S, Ceresoli, et al.: Efficacy and tolerability of gefitinib in pretreated elderly patients with advanced nonsmall-cell lung cancer (NSCLC). Br J Cancer 2004, 90:826–886.
Johnson B, Lucca J, Rabin M, et al.: Preliminary results from a phase II study of the epidermal growth factor receptor tyrosine kinase inhibitor erlotinib in patients > 70 years of age with previously untreated advanced non-small cell lung carcinoma [abstract]. Proc ASCO 2004, 23:7080.
Hotta K, Kiura K, Ueoka, et al.: Effect of gefitinib (‘Iressa’, ZD1839) on brain metastases in patients with advanced nonsmall-cell lung cancer. Lung Cancer 2004, 46:255–261.
Ceresoli G, Cappuzzo F, Gregorc V, et al.: Gefitinib in patients with brain metastases from non-small-cell lung cancer: a prospective trial. Ann Oncol 2004, 15:1042–1047.
Chiu CH, Tsai CM, Chen YM, et al.: Gefitinib is active in patients with brain metastases from non-small cell lung cancer and response is related to skin toxicity. Lung Cancer 2005, 47:123–138.
Lynch TJ, Lilenbaum R, Bonomi P, et al.: A phase II trial of cetuximab as therapy for recurrent non-small cell lung cancer (NSCLC) [abstract]. Proc ASCO 2004, 23:7084.
Rosell R, Daniel C, Ramlau R, et al.: Randomized phase II study of cetuximab in combination with cisplatin (C) and vinorelbine (V) vs. CV alone in the first-line treatment of patients (pts) with epidermal growth factor receptor (EGFR)-expressing advanced non-small cell lung cancer (NSCLC) [abstract]. Proc ASCO 2004, 23:7012.
Kim ES, Mauer Am, Tran HT, et al.: A phase II study of cetuximab, an epidermal growth factor receptor (EGFR) blocking antibody, in combination with docetaxel in chemotherapy refractory/resistant patients with advanced non-small cell lung cancer: final report [abstract]. Proc ASCO 2003, 22:2581.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Byrne, B.J., Garst, J. Epidermal growth factor receptor inhibitors and their role in non-small-cell lung cancer. Curr Oncol Rep 7, 241–247 (2005). https://doi.org/10.1007/s11912-005-0045-6
Issue Date:
DOI: https://doi.org/10.1007/s11912-005-0045-6