Abstract
Purpose of review
Rapid eye movement (REM) sleep behaviour disorder (RBD) is considered the expression of the initial neurodegenerative process underlying synucleinopathies and constitutes the most important marker of their prodromal phase. This article reviews recent research from longitudinal research studies in isolated RBD (iRBD) aiming to describe the most promising progression biomarkers of iRBD and to delineate the current knowledge on the level of prediction of future outcome in iRBD patients at diagnosis.
Recent findings
Longitudinal studies revealed the potential value of a variety of biomarkers, including clinical markers of motor, autonomic, cognitive, and olfactory symptoms, neurophysiological markers such as REM sleep without atonia and electroencephalography, genetic and epigenetic markers, cerebrospinal fluid and serum markers, and neuroimaging markers to track the progression and predict phenoconversion. To-date the most promising neuroimaging biomarker in iRBD to aid the prediction of phenoconversion is striatal presynaptic striatal dopaminergic dysfunction.
Summary
There is a variety of potential biomarkers for monitoring disease progression and predicting iRBD conversion into synucleinopathies. A combined multimodal biomarker model could offer a more sensitive and specific tool. Further longitudinal studies are warranted to iRBD as a high-risk population for early neuroprotective interventions and disease-modifying therapies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rapid eye movement (REM) sleep behaviour disorder (RBD) is a parasomnia clinically characterized by active dream enactment, including screaming, flinging, or falling off from bed, that may cause injuries to patients and their bed partners [1]. According to the American Academy of Sleep Medicine, the diagnosis of RBD relies on the confirmation, with video-polysomnography (PSG), of decreased muscle atonia and sudden movements during sleep [2]. RBD is considered a common disorder. The pooled prevalence of PSG-confirmed RBD has been estimated at 0.68% of the general population, and that of probable RBD at 5.65% [3].
Isolated RBD (iRBD) is now considered the expression of the initial neurodegenerative process underlying synucleinopathies, including Parkinson’s disease (PD), Dementia with Lewy Bodies (DLB) and Multiple System Atrophy (MSA) [4] and constitutes the most important marker of the prodromal phase of these neurodegenerative diseases [5]. Large longitudinal cohort studies have demonstrated that 81–91% of iRBD patients, followed-up for at least 14 years, will develop either a definite neurodegenerative disease or a mild cognitive impairment [6, 7].
iRBD is considered a marker of neurodegeneration with strong predictive value and scarce sensitivity. The likelihood ratio of PSG-proven iRBD for development of a synucleinopathy has been estimated at 130, more than three times higher than that of detecting striatal dopamine loss on molecular imaging [8]. By contrast, only about 50% of all PD patients have experienced iRBD in their prodromal stage [9].
In the future, iRBD could be considered a condition which is useful to be targeted with new disease-modifying therapies, currently in development aiming to interrupt the pathological processes towards the development of synucleinopathies at an earlier stage. The study of iRBD pathology can allow the understanding of biological alterations preceding the clinical manifestation of a synucleinopathy, thus anticipating personalized treatments at a stage where cellular damage could be reversible [10]. To do so, we need to understand which characteristics of iRBD are associated with pathological progression, and with faster or slower phenoconversion [11]. The past few years have seen an increase of longitudinal studies aimed at establishing the value of multiple clinical, genetic, neurophysiological, fluid, and imaging biomarkers in the prediction of the progression from iRBD to PD, DLB, and MSA [12].
This article reviews the most relevant results from recent longitudinal research studies in iRBD, with the intent of describing the most promising progression biomarkers of iRBD and to delineate the current knowledge on the level of prediction in iRBD patients at diagnosis and their future outcome (Table 1).
Clinical Markers of Progression
Prodromal PD is a clinical entity characterized by the presence of motor and non-motor symptoms, such as alterations in motor dexterity, autonomic dysfunction, mood, olfaction, and cognition, that reflect the progressing neuronal damage in the brain [50]. Many of these clinical symptoms co-occur in iRBD and have been extensively studied in large, multicentre, longitudinal studies to assess whether they could represent a sign of progressing degeneration and a predictor of short-term diagnosis of a neurodegenerative disease.
Motor
An akinetic-rigid syndrome is a hallmark feature of synucleinopathies and current dopaminergic therapy is principally directed at improving these symptoms. In future PD converters with iRBD, performance on tasks assessing motor dexterity can be altered as early as 12.9 years from diagnosis [13••]. Additionally, an increase in the Unified Parkinson’s disease Rating Scale part III (UPDRS-III) score is first detected at around 6.5 years before diagnosis and accelerates in the final 1–2 years before that [13••]. The increase of UPDRS-III score is initially driven by speech and voice alterations, followed in time by bradykinesia, rigidity and, lastly, rest tremor [13••]. In a large multicentric study performed by Postuma and colleagues, the hazard ratio of both UPDRS-III score and of performance on quantitative motor tests was comparable, in entity, to that of altered striatal uptake on [123I]FP-CIT SPECT, a marker of presynaptic dopamine transporter (DAT) availability [14••]. In iRBD patients converting to DLB, motor symptoms appear earlier than in PD converters but, differently from the latter, they progress at a slower pace [14••]. Overall, alterations in motor performances yield similar degrees of prediction towards future development of either dementia or parkinsonism [14••].
Autonomic
Up to 94% of iRBD patients report symptoms of autonomic dysfunction [51]. Studies of autonomic function in iRBD have employed specific scales and questionnaires (such as Scales for Outcomes in PD—Autonomic Dysfunction (SCOPA-AUT) and the Non-Motor Symptoms Questionnaire (NMSQ)) as well as instrumental tests (heart rate variability, cardiovascular reflex testing, cardiac scintigraphy, etc.) to assess autonomic alteration [52]. Symptoms due to sympathetic dysfunction in iRBD can be detected through administration of clinical scales as early as 16 years before clinical diagnosis and total scores of these scales become statistically different from controls at around 4–6 years from diagnosis [13••].
High scores on specific autonomic symptoms are found more frequently in iRBD converting to a specific synucleinopathy. MSA converters show more severe urinary symptoms, whereas DLB converters show faster declines of systolic blood pressure. In a recent small study on 18 iRBD patients, in which the severity of alterations of preganglionic and postganglionic sudomotor, cardiovagal, and cardiovascular adrenergic function on instrumental tests was converted to a composite score (CASS score), it was found that the iRBD who converted to DLB had a longer duration of autonomic dysfunction and a higher degree of impairment of cardiovagal and, to a lesser degree, adrenergic autonomic dysfunction, compared to those who converted to PD [15].
A decreased heart rate variability (HRV) recorded on full-night PSG is an early feature of prodromal PD [53] and is also found in iRBD patients [16]. Alterations in beat-to-beat variability in a cohort of iRBD patients studied longitudinally for an average 6.7 years, however, did not discriminate between patients who eventually converted from those who did not [17]. Very recently, presence of low HRV in a cohort of 47 iRBD was associated with severity of the quantified tonic REM Sleep without Atonia (RSWA), an electrophysiological marker of severity of RBD and possible predictor of phenoconversion [18].
Cognitive Dysfunction
iRBD patients frequently display cognitive dysfunction [54] which progresses over time [55, 56, 57]. In turn, about 35% of patients with Mild Cognitive Impairment (MCI) have iRBD [58]. The most affected cognitive domains in iRBD are attention, executive functions, and visuospatial abilities [19–21, 58••, 59, 60••]. Dysfunction of these cognitive abilities in iRBD patients has been associated with faster conversion to a neurodegenerative disease in studies with short follow-up (fewer than three years) [19, 21]. On the basis of clinical, neuroimaging, and neurophysiological findings, it has also been suggested that iRBD plus MCI may represent a distinct, more aggressive phenotype that iRBD alone [21, 60••, 61, 62, 63].
A number of recent studies have investigated the cognitive profile, and the trajectory of cognitive impairment progression in iRBD patients who eventually convert to DLB, as opposed to PD. DLB converters examined up to six years before diagnosis, already show alterations on attention, executive function, and verbal memory with subsequent development of deficits in episodic verbal learning and memory and a faster progression compared to PD converters [13••, 19, 20]. PD converters, by contrast, display cognitive performances within normal limits until 1–2 years before diagnosis [19]. Overall, presence at baseline of multidomain cognitive dysfunction in iRBD patients is the main clinical characteristics able to predict whether a patient will end up developing DLB or PD [13••].
Hyposmia
Olfactory impairment is a frequent symptom in iRBD [64]. Odour identification tests have been widely employed in clinical studies to evaluate olfactory impairment in iRBD patients. Alterations in odour identification scores can be spotted, in iRBD patients, as early as 22 years before diagnosis of a neurodegenerative condition and become significantly impaired compared with controls nine years before phenoconversion [13••]. Hyposmia in iRBD, however, does not seem to progress at a faster pace than in normal ageing [13••, 22].
Alterations of odour identification has been linked with a 7.3-fold increased risk of developing a synucleinopathy within five years [24]. These results have been replicated in a recent larger study of 140 iRBD patients that underwent odour identification test and were followed up for an average 5.6 years. Here, hyposmia was associated to a higher risk of developing either PD or DLB in the short term, without however discriminating prospectively between the two conditions [22].
Studies on MSA converters are hindered by the small sample sizes but suggest that olfactory dysfunction at baseline does not predict the future conversion to MSA. In one study of twelve iRBD patients tested four years before conversion to MSA, hyposmia was present in 50%, a percentage higher than controls but significantly lower than in PD [25]. In the study by Iranzo and colleagues, the three iRBD patients eventually diagnosed with MSA after follow-up were all normosmic [22]. These findings are consistent with the low prevalence of olfactory dysfunction in the clinical picture of MSA [23].
Visual Dysfunction
Patients with iRBD exhibit different degrees of visual dysfunction. These encompass abnormal colour discrimination and stereopsis and illusions [65]. Studies on visual dysfunction have employed a range of tests, from contrast sensitivity tests to colour vision discrimination tests. Abnormal colour vision in iRBD is associated with a higher risk of developing a neurodegenerative synucleinopathy [26]. Colour vision testing performed at baseline can identify iRBD patients who will later convert to DLB as opposed to those who will later convert to PD [14••]. In addition, the trajectory of colour vision impairment in DLB converters progression is steeper than that of PD converters [14••]. However, the clinical test used to assess colour vision discrimination has a visuoperceptual cognitive component that may bias this result [66].
In a recent small study, the visual acuity and the contrast sensitivity of 12 iRBD has been found to be reduced compared to controls, and further declined after a one-year follow-up [67]. Further tests would be needed to assess whether this could constitute a possible marker of progression in iRBD.
Genetic Markers of Progression
Around 5–10% of all PD cases can be ascribed to single gene mutations. In the last twenty years, several rare, highly-penetrant mutations with Mendelian inheritance, as well as frequent variants with smaller effects, have been discovered [68]. Mutations of the GBA gene, encoding for Glucocerebrosidase, are associated with higher risk of PD and DLB [69, 70, 30]. In these cases, the frequency of RBD is higher than in non-GBA cases, and the severity of the phenotype is influenced by the type of the GBA mutation [71]. Patients with iRBD display higher frequency of GBA mutations compared to healthy controls, and comparable to that of PD patients [27, 72, 73, 28]. Within iRBD patients, GBA mutation carriers tend to have an earlier age at onset, but do not present any other distinctive phenotypic characteristics compared to iRBD patients negative for GBA mutations [28].
Three recent studies have attempted to establish whether GBA mutations in iRBD confer with higher risk of phenoconversion. In one study with 8 iRBD GBA carriers, no such association was detected [27]. In another study with 13 iRBD with GBA mutations, a 3.2-fold higher rate of phenoconversion towards parkinsonism and/or dementia was detected [28]. In 2020, Krohn and colleagues gathered a large multicentre longitudinal database of 1061 patients with iRBD, of which 9.5% carried a GBA mutation, and stratified them according to severity of the gene variants. These Authors found that severe variants (L444P, D409H, W291X, H255Q, and R131L) were associated with higher risk for iRBD compared to the mild N370S variant. Additionally, there was a trend for severe variants to drive towards faster conversion to a neurodegenerative disease [29••]. However, the number of severe variant carriers was very low and further studies are needed to confirm this finding.
Recently, mutations in the TMEM175 gene have gained academic attention for their relationship with PD risk [74]. Krohn and colleagues have identified the p.M393T variant on TMEM175 as strongly associated with the risk of both PD and iRBD [75]. The p.Q65P variant was then associated with an increased rate of phenoconversion to a synucleinopathy [31••]. Recent cross-sectional studies on large cohorts have also detected genetic associations between iRBD risk and variants in the genes SNCA, BST1, and LAMP3, which could further expand our knowledge on the links between genetics and development and progression of iRBD [76, 77].
Epigenetic mechanisms have also been recently studied in relation to their progression risk from iRBD to neurodegeneration. In a recent small, preliminary study on 78 patients with iRBD of which 16 converted to PD after 3.75 years, hypomethylation at the Cytosine-phosphate-Guanine (CpG) 17 of the SNCA intron 1 has been associated with increased risk of clinical phenoconversion, and hypomethylation to the CpG 14, 15, and 16 was associated with progression of iRBD symptoms [32•].
Neurophysiological Markers of Neurodegeneration
Electrophysiology is an essential tool to diagnose and characterize iRBD and has long been employed to identify changes in sleep structure and brain electrical activity with potential to predict evolution of iRBD into a neurodegenerative disease. Various patterns of REM and non-REM sleep, and wake activity have been studied in relation to disease severity and progression, and to their prediction of conversion to a synucleinopathy [78].
REM Sleep without Atonia
The finding of an abnormal electromyographic activity on PSG during REM sleep is denominated REM Sleep Without Atonia (RSWA) and is a pathognomonic feature of RBD. According to its characteristics, RSWA can be tonic, or phasic. The severity of RSWA in iRBD increases over time and this arguably reflects the progression of the brainstem damage induced by the neurodegenerative process [79]. The percentage of tonic RSWA at baseline, in iRBD, has been established as a strong predictor of future conversion to PD [33].
Tonic and phasic RSWA are thought to represent the electrophysiological expression of different pathophysiological alterations taking place in the brainstem [80, 81]. Recent studies have focused on the possible different predictive role of either tonic or phasic RSWA towards neurodegeneration. One large study assessed 216 patients with iRBD who were followed-up for five years, and 26.9% of these iRBD patients developed a neurodegenerative disease [34•]. Baseline tonic RSWA showed a stable predictive capacity of future development of PD over time, whereas baseline phasic RSWA was only predictive of future conversion to DLB at long follow-up [34•]. This was confirmed in another recent study in which percentage of tonic RSWA was predictive of a more rapid conversion to parkinsonism, but not of cognitive impairment, thus suggesting that distinction between RSWA subtypes could predict future development of neurodegeneration in iRBD patients [35].
Isolated RSWA (iRSWA) is the detection of RSWA in absence of other symptoms ascribable to RBD [82]. It can be an incidental finding in up of 5% of PSG, and its frequency increases with age. iRSWA can be associated with other electrophysiological, clinical, imaging, or autonomic findings [16, 83, 36, 84]. A few studies have tested the hypothesis that iRSWA could represent an initial manifestation of neurodegeneration, yielding however conflicting results. Stefani and colleagues did not report any progression of iRSWA patients towards neurodegeneration after a 8.6-year follow-up [36], and in another study, iRSWA was not correlated with striatal dopamine levels as assessed with [123I]FP-CIT SPECT [37]. By contrast, Dede and colleagues, studying 67 iRSWA patients for at least 4 years, reported that 26.8% developed RBD and 8.9% developed a neurodegenerative disorder. This study, however, lacked a control group to ascertain whether the progression was due to aging or by a genuine increased risk of iRSWA [38].
Electroencephalography
Electroencephalography (EEG) studies in iRBD show a diffuse slowness of cortical activity [85], which correlates with cognitive tests exploring attention, executive functions, and verbal memory [86, 39]. Two longitudinal studies have assessed the predictive value of EEG alterations in iRBD towards neurodegeneration. One study enrolled 54 iRBD patients to perform quantitative EEG and to a 3.5-year follow-up. The iRBD patients who converted after follow-up showed higher δ and θ power in the cortex, with higher slow-to-fast power ratio. Most importantly, detection of diffuse cortical EEG slowing was predictive of conversion to DLB, whereas EEG slowing restricted to temporal and occipital lobes was predictive of conversion to PD [39]. In a second study on 121 patients with iRBD, of which 27 converted to either PD or DLB after four years, diffuse bursts of θ band together with a decrease of bursting in the α band could distinguish iRBD converters compared to controls [40].
EEG during non-REM sleep has also been studied as potential marker of neurodegeneration in iRBD. Cyclic Alternating Pattern (CAP) is a spontaneous, physiological rhythm of non-REM sleep composed of transient electro-cortical events of arousal, followed by retrieval to background EEG activity that is interpreted as an expression of arousal instability [87, 88]. The number and architecture of CAP is significantly altered in iRBD [89]. Melpignano and colleagues studied 67 iRBD patients and found that CAP cycles were longer, and their rate significantly decreased. In addition, they found that CAP rate was most reduced in those patients who converted earlier to a neurodegenerative disease [41]. Further, confirmatory studies will establish the potential of microstructural alterations of non-REM sleep architecture as potential markers of progression of neurodegeneration in iRBD.
Fluid Biomarkers
Fluid biomarkers including cerebrospinal fluid (CSF) markers, such as oligomeric, total and phosphorylated α-synuclein, total and phosphorylated tau, amyloid-β42 and neurofilament light chain, have become increasingly investigated as a source of potential biomarkers providing insights into the pathogenesis of neurodegenerative diseases. PD patients with RBD have been shown to have higher CSF and serum levels of oligomeric α-synuclein compared to PD patients without RBD [90]. Furthermore, the presence of RBD in PD patients has shown to be a predictor of motor progression in patients with both low α-synuclein CSF levels and reduced striatal DAT [123I]FP-CIT uptake, and a predictor of cognitive decline in patients with low CSF levels of both α-synuclein and low amyloid-β42 [91]. A longitudinal study illustrated that lower baseline amyloid-β42 levels were predictive of cognitive decline at three-year follow-up only in PD patients with RBD [92]. Increased CSF prion protein levels have also been reported in PD patients with RBD compared to PD patients without RBD [93]. CSF inflammatory markers, including interleukin 1β and nitric oxide, as well as serum prostaglandin E2 have also been shown to be elevated in PD patients with RBD [90]. A recent study in probable iRBD patients illustrated that a reduced ratio of phosphorylated tau to total tau was associated with phenoconversion to a synucleinopathy disease at a 5-year follow-up highlighting the potential use of fluid biomarkers to track progression in iRBD [31••]. Future studies investigating fluid biomarkers in iRBD patients, prior to the clinically diagnosis of a synucleinopathy disease, are warranted to fully elucidate the potential utility of CSF and blood biomarkers to monitor the progression of RBD.
Neuroimaging Biomarkers
The last decade has seen an increasing volume of neuroimaging studies, employing Positron Emission Tomography (PET), Single Photon Emission Computed Tomography (SPECT), Magnetic Resonance Imaging (MRI) and transcranial sonography techniques, to investigate the pathophysiology of iRBD and to help identify potential biomarkers to predict the progression of RBD and the conversion of iRBD to a synucleinopathy disease.
[123I]FP-CIT SPECT and transcranial sonography have been shown to detect subclinical changes in iRBD patients, similar to pathology seen in early PD [42–44]. Four longitudinal studies have used dopaminergic SPECT to investigate the progression of presynaptic striatal dopamine pathology as a biomarker in iRBD patients [42–45]. Iranzo and colleagues demonstrated that lower striatal presynaptic DAT availability, using [123I]FP-CIT SPECT, combined with hyperechogenicity of the substantia nigra, using transcranial sonography, had a predictive value of 100%, with 55% specificity, after 2.5 years to predict the conversion of iRBD patients to a neurodegenerative synucleinopathy [44]. Repeated [123I]FP-CIT SPECT scans show progressive loss of striatal DAT in iRBD patients over three years, with iRBD patients who converted to PD showing the greatest level of nigrostriatal dopaminergic dysfunction at baseline [42]. Furthermore, a reduction of [123I]FP-CIT SPECT greater than 25% in the putamen has been shown to discriminate iRBD patients, with DAT deficits, who converted to a synucleinopathy from iRBD patients who did not convert after three-year follow-up [43]. At a five-year follow-up [123I], FP-CIT SPECT had 75% sensitivity and 51% specificity to predict iRBD conversion to a synucleinopathy with a likelihood ratio of 1.54 [43]. Li and colleagues further highlighted the predictive value of decreased DAT in the putamen and striatum, using [99mTC]TRODAT-1 SPECT, in iRBD patients over 5 years with greater DAT deficits in those patients at high risk of progressing to a synucleinopathy [45]. Together these studies provide evidence to suggest that presynaptic nigrostriatal dopaminergic dysfunction, detected using SPECT imaging, could offer a valuable biomarker to monitor the progression of nigrostriatal deficits in RBD patients with the potential ability to aid the prediction of phenoconversion to a neurodegenerative synucleinopathy. DAT SPECT imaging is already used in clinical practice [94] therefore the platform to implement this tool in iRBD could be feasible. To aid potential translation into clinical practice, large, multicentre, longitudinal studies are warranted to further validate the use of SPECT imaging as a tool to predict phenoconversion in iRBD patients.
Glucose metabolism and perfusion changes have been reported in iRBD patients with spatial covariance analysis identifying abnormal PD-related metabolic brain networks [95, 96, 97, 98]. A longitudinal [99mTc]ECD SPECT study illustrated increased perfusion in the hippocampus of RBD patients who developed a synucleinopathy at a 3-year follow-up compared to those who did not progress [46]. A logistical regression model combing increased PD-related covariance pattern expression with age was predictive of phenoconversion from iRBD to a synucleinopathy at an average follow-up of 4.6 years [47]. Furthermore, recent work by Kogan and colleagues supports the potential use of multiple [18F]FDG PET scans to measure progressive changes in PD-related brain pattern expression measures as a prodromal PD biomarker to predict phenoconversion in iRBD patients [48••].
Cardiac [123I]metaiodobenzylguanidine (MIBG) scintigraphy has also been investigated in iRBD patients. While cross-sectional studies have revealed abnormalities [99, 100, 101], a longitudinal study reported no changes in RBD patients at a 2.5-year follow-up [102]. These findings suggest that while sympathetic denervation may be abnormal in early RBD patients, [123I]MIBG might not be a sensitive biomarkers for the progression and phenoconversion in RBD patients.
Structural and functional MRI techniques have demonstrated changes in deep grey matter, cortical grey matter, microstructural white matter and disrupted functional connectivity networks in patients with RBD which can be associated with clinical symptoms [103]. Isolated RBD patients who converted to a clinically defined synucleinopathy, at a three-year follow-up, showed greater cortical thinning in frontal, parietal and occipital cortices compared to iRBD patients who did not convert [49••]. Pereira and colleagues reported cortical thinning as a predictor of phenoconversion in iRBD [49••]. Furthermore, grey matter atrophy in the inferior frontal gyrus has been associated with phenoconversion at 5-year follow-up [31••]. Together these studies suggest that structural neuroimaging could act as a predictive biomarker for increased risk of progression to a synucleinopathy.
Diffusion-weighted MRI has been employed to define longitudinal brain connectome progression scores, using interpretable machine learning algorithm, to evaluate the progression patterns in iRBD patients as a prodromal phase of PD [104•]. The longitudinal connectome progression pattern in iRBD patients was similar to that of de novo PD patients, highlighting the potential of this tool as a biomarker for the neurodegenerative prodromal phase of synucleinopathies [104•]. This study highlights the potential future use of MRI-based computational biomarkers to predict the progression and conversion of RBD with high sensitivity and specificity. Longitudinal studies, such as the Oxford PD Centre Discovery Cohort MRI substudy (OPDC-MRI) [105], are ongoing to validate the use of structural and functional MRI techniques, combined with clinical data, as biomarkers to predict the progression and phenoconversion of iRBD to synucleinopathies.
Conclusion
As a high-risk population for conversion to synucleinopathies, iRBD offers a valuable therapeutic window for application of early neuroprotective interventions and disease-modifying therapies. Recent longitudinal studies have highlighted a variety of potential biomarkers, including clinical, neurophysiological, genetic, CSF, serum, and neuroimaging, for monitoring disease progression and predicting iRBD conversion into synucleinopathies (Fig. 1). However, the role of biomarkers as predictors of iRBD remains to be fully elucidated. A combined multimodal biomarker model could offer a sensitive and specific tool to predict the progression of RBD and conversion to synucleinopathies. Future studies are required, most notably large, multicentre, longitudinal studies, to validate these potential biomarkers and step towards their use as endpoints in future clinical trials.
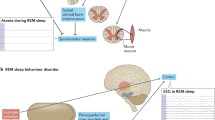
Schematic summary of the main areas of biological alterations found in association with increased rate of progression and/or phenoconversion of iRBD patients to synucleinopathy. Abbreviations: EEG: electroencephalogram; GBA: glucocerebrosidase; REM: rapid eye movements; SNCA: synuclein; TMEM175: transmembrane protein 175
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Dauvilliers Y, Schenck CH, Postuma RB, Iranzo A, Luppi PH, Plazzi G, et al. REM sleep behaviour disorder. Nat Rev Dis Primers. 2018;4(1):19. https://doi.org/10.1038/s41572-018-0016-5.
Sateia MJ. International classification of sleep disorders-third edition: highlights and modifications. Chest. 2014;146(5):1387–94. https://doi.org/10.1378/chest.14-0970.
Cicero CE, Giuliano L, Luna J, Zappia M, Preux PM, Nicoletti A. Prevalence of idiopathic REM behavior disorder: a systematic review and meta-analysis. Sleep. 2021;44(6). doi:https://doi.org/10.1093/sleep/zsaa294.
Iranzo A, Santamaria J, Tolosa E. Idiopathic rapid eye movement sleep behaviour disorder: diagnosis, management, and the need for neuroprotective interventions. Lancet Neurol. 2016;15(4):405–19. https://doi.org/10.1016/S1474-4422(16)00057-0.
Berg D, Borghammer P, Fereshtehnejad SM, Heinzel S, Horsager J, Schaeffer E, et al. Prodromal Parkinson disease subtypes - key to understanding heterogeneity. Nat Rev Neurol. 2021;17(6):349–61. https://doi.org/10.1038/s41582-021-00486-9.
Schenck CH, Boeve BF, Mahowald MW. Delayed emergence of a parkinsonian disorder or dementia in 81% of older men initially diagnosed with idiopathic rapid eye movement sleep behavior disorder: a 16-year update on a previously reported series. Sleep Med. 2013;14(8):744–8. https://doi.org/10.1016/j.sleep.2012.10.009.
Iranzo A, Fernandez-Arcos A, Tolosa E, Serradell M, Molinuevo JL, Valldeoriola F, et al. Neurodegenerative disorder risk in idiopathic REM sleep behavior disorder: study in 174 patients. PLoS ONE. 2014;9(2): e89741. https://doi.org/10.1371/journal.pone.0089741.
Berg D, Postuma RB, Adler CH, Bloem BR, Chan P, Dubois B, et al. MDS research criteria for prodromal Parkinson’s disease. Mov Disord. 2015;30(12):1600–11. https://doi.org/10.1002/mds.26431.
Gagnon JF, Bedard MA, Fantini ML, Petit D, Panisset M, Rompre S, et al. REM sleep behavior disorder and REM sleep without atonia in Parkinson’s disease. Neurology. 2002;59(4):585–9. https://doi.org/10.1212/wnl.59.4.585.
Lin Y, Qian F, Shen L, Chen F, Chen J, Shen B. Computer-aided biomarker discovery for precision medicine: data resources, models and applications. Brief Bioinform. 2019;20(3):952–75. https://doi.org/10.1093/bib/bbx158.
Cova I, Priori A. Diagnostic biomarkers for Parkinson’s disease at a glance: where are we? J Neural Transm (Vienna). 2018;125(10):1417–32. https://doi.org/10.1007/s00702-018-1910-4.
Crosiers D, Santens P, Chaudhuri KR. Editorial: Prodromal Parkinson’s Disease. Front Neurol. 2020;11: 634490. https://doi.org/10.3389/fneur.2020.634490.
•• Fereshtehnejad SM, Yao C, Pelletier A, Montplaisir JY, Gagnon JF, Postuma RB. Evolution of prodromal Parkinson’s disease and dementia with Lewy bodies: a prospective study. Brain. 2019;142(7):2051–67. https://doi.org/10.1093/brain/awz111. (This study makes a large, longitudinal, prospective description of the trajectory of clinical symptoms of a large cohort of iRBD patients from diagnosis up to phenoconversion to a synucleinopathy.)
•• Postuma RB, Iranzo A, Hu M, Hogl B, Boeve BF, Manni R, et al. Risk and predictors of dementia and parkinsonism in idiopathic REM sleep behaviour disorder: a multicentre study. Brain. 2019;142(3):744–59. https://doi.org/10.1093/brain/awz030. (This study on a large cohort of iRBD patients provides a quantitative estimate of risk of a large number of clinical sleep, motor, cognitive, autonomic and special sensory biomarkers towards phenoconversion to a synucleinopathy on a follow-up of 12 years.)
McCarter SJ, Gehrking TL, St Louis EK, Suarez MD, Boeve BF, Silber MH, et al. Autonomic dysfunction and phenoconversion in idiopathic REM sleep behavior disorder. Clin Auton Res. 2020;30(3):207–13. https://doi.org/10.1007/s10286-020-00674-5.
Barone DA, Ebben MR, Samie A, Mortara D, Krieger AC. Autonomic dysfunction in isolated rapid eye movement sleep without atonia. Clin Neurophysiol. 2015;126(4):731–5. https://doi.org/10.1016/j.clinph.2014.07.015.
Postuma RB, Lanfranchi PA, Blais H, Gagnon JF, Montplaisir JY. Cardiac autonomic dysfunction in idiopathic REM sleep behavior disorder. Mov Disord. 2010;25(14):2304–10. https://doi.org/10.1002/mds.23347.
Yang JH, Choi SH, Lee MH, Oh SM, Choi JW, Park JE, et al. Association of heart rate variability with REM sleep without atonia in idiopathic REM sleep behavior disorder. J Clin Sleep Med. 2021;17(3):461–9. https://doi.org/10.5664/jcsm.8934.
Genier Marchand D, Montplaisir J, Postuma RB, Rahayel S, Gagnon JF. Detecting the Cognitive Prodrome of Dementia with Lewy Bodies: A Prospective Study of REM Sleep Behavior Disorder. Sleep. 2017;40(1). doi:https://doi.org/10.1093/sleep/zsw014.
Genier Marchand D, Postuma RB, Escudier F, De Roy J, Pelletier A, Montplaisir J, et al. How does dementia with Lewy bodies start? prodromal cognitive changes in REM sleep behavior disorder. Ann Neurol. 2018;83(5):1016–26. https://doi.org/10.1002/ana.25239.
Terzaghi M, Toscano G, Casoni F, Picascia M, Arnaldi D, Rustioni V et al. Assessment of cognitive profile as a prodromal marker of the evolution of rapid eye movement sleep behavior disorder. Sleep. 2019;42(8). doi:https://doi.org/10.1093/sleep/zsz103.
Iranzo A, Marrero-Gonzalez P, Serradell M, Gaig C, Santamaria J, Vilaseca I. Significance of hyposmia in isolated REM sleep behavior disorder. J Neurol. 2021;268(3):963–6. https://doi.org/10.1007/s00415-020-10229-3.
Wenning GK, Shephard B, Hawkes C, Petruckevitch A, Lees A, Quinn N. Olfactory function in atypical parkinsonian syndromes. Acta Neurol Scand. 1995;91(4):247–50. https://doi.org/10.1111/j.1600-0404.1995.tb06998.x.
Mahlknecht P, Iranzo A, Hogl B, Frauscher B, Muller C, Santamaria J, et al. Olfactory dysfunction predicts early transition to a Lewy body disease in idiopathic RBD. Neurology. 2015;84(7):654–8. https://doi.org/10.1212/WNL.0000000000001265.
Stefani A, Ferini-Strambi L, Postuma RB, Iranzo A, Videnovic A, Hogl B et al. Olfaction in patients with isolated REM sleep behavior disorder who eventually develop multiple system atrophy. Sleep. 2020;43(4). doi:https://doi.org/10.1093/sleep/zsz303.
Postuma RB, Gagnon JF, Bertrand JA, Genier Marchand D, Montplaisir JY. Parkinson risk in idiopathic REM sleep behavior disorder: preparing for neuroprotective trials. Neurology. 2015;84(11):1104–13. https://doi.org/10.1212/WNL.0000000000001364.
Gamez-Valero A, Iranzo A, Serradell M, Vilas D, Santamaria J, Gaig C, et al. Glucocerebrosidase gene variants are accumulated in idiopathic REM sleep behavior disorder. Parkinsonism Relat Disord. 2018;50:94–8. https://doi.org/10.1016/j.parkreldis.2018.02.034.
Honeycutt L, Montplaisir JY, Gagnon JF, Ruskey J, Pelletier A, Gan-Or Z, et al. Glucocerebrosidase mutations and phenoconversion of REM sleep behavior disorder to parkinsonism and dementia. Parkinsonism Relat Disord. 2019;65:230–3. https://doi.org/10.1016/j.parkreldis.2019.04.016.
•• Krohn L, Ruskey JA, Rudakou U, Leveille E, Asayesh F, Hu MTM, et al. GBA variants in REM sleep behavior disorder: A multicenter study. Neurology. 2020;95(8):e1008–16. https://doi.org/10.1212/WNL.0000000000010042. (This article describes the role of different GBA mutations in the risk of phenoconversion from iRBD to a synucleinopathy in a large multicentre cohort of iRBD patients.)
Nalls MA, Duran R, Lopez G, Kurzawa-Akanbi M, McKeith IG, Chinnery PF, et al. A multicenter study of glucocerebrosidase mutations in dementia with Lewy bodies. JAMA Neurol. 2013;70(6):727–35. https://doi.org/10.1001/jamaneurol.2013.1925.
•• Ye G, Li Y, Zhou L, Zhang Y, Zhu L, Zhao A, et al. Predictors of Conversion to alpha-Synucleinopathy Diseases in Idiopathic Rapid Eye Movement Sleep Behavior Disorder. J Parkinsons Dis. 2020;10(4):1443–55. https://doi.org/10.3233/JPD-202243. (In this 5-year longitudinal study, a model combining three independent variables, clinical marker (UPSIT), genotype status (TMEM175), and structural MRI marker (grey matter atrophy), showed good accuracy for the prediction of iRBD patients progression to a synucleinopathy.)
• Li Y, Hao S, Zhang H, Mao W, Xue J, Zhang Y, et al. Hypomethylation of SNCA in Idiopathic REM Sleep Behavior Disorder Associated With Phenoconversion. Mov Disord. 2021;36(4):955–62. https://doi.org/10.1002/mds.28421. (This article broadens the range of genetic and epigenetic alterations in the influence of the progression from iRBD to synucleinopathy.)
Postuma RB, Gagnon JF, Rompre S, Montplaisir JY. Severity of REM atonia loss in idiopathic REM sleep behavior disorder predicts Parkinson disease. Neurology. 2010;74(3):239–44. https://doi.org/10.1212/WNL.0b013e3181ca0166.
• Liu Y, Zhang J, Lam SP, Yu MWM, Li SX, Zhou J, et al. Electromyography activity level in rapid eye movement sleep predicts neurodegenerative diseases in idiopathic rapid eye movement sleep behavior disorder: a 5-year longitudinal study. Sleep Med. 2019;56:128–34. https://doi.org/10.1016/j.sleep.2019.01.018. (This article demonstrated that tonic and phasic RSWA underlie distinct phenotypes of progression in iRBD, highlighting their different pathophysiological significance.)
McCarter SJ, Tabatabai GM, Jong HY, Sandness DJ, Timm PC, Johnson KL, et al. REM sleep atonia loss distinguishes synucleinopathy in older adults with cognitive impairment. Neurology. 2020;94(1):e15–29. https://doi.org/10.1212/WNL.0000000000008694.
Stefani A, Gabelia D, Hogl B, Mitterling T, Mahlknecht P, Stockner H, et al. Long-Term Follow-up Investigation of Isolated Rapid Eye Movement Sleep Without Atonia Without Rapid Eye Movement Sleep Behavior Disorder: A Pilot Study. J Clin Sleep Med. 2015;11(11):1273–9. https://doi.org/10.5664/jcsm.5184.
Fujishiro H, Okuda M, Iwamoto K, Miyata S, Torii Y, Iritani S, et al. Early diagnosis of Lewy body disease in patients with late-onset psychiatric disorders using clinical history of rapid eye movement sleep behavior disorder and [(123) I]-metaiodobenzylguanidine cardiac scintigraphy. Psychiatry Clin Neurosci. 2018;72(6):423–34. https://doi.org/10.1111/pcn.12651.
Dede HO, Benbir Senel G, Karadeniz D. Rapid eye movement sleep without atonia constitutes increased risk for neurodegenerative disorders. Acta Neurol Scand. 2019;140(6):399–404. https://doi.org/10.1111/ane.13156.
Rodrigues Brazete J, Montplaisir J, Petit D, Postuma RB, Bertrand JA, Genier Marchand D, et al. Electroencephalogram slowing in rapid eye movement sleep behavior disorder is associated with mild cognitive impairment. Sleep Med. 2013;14(11):1059–63. https://doi.org/10.1016/j.sleep.2013.06.013.
Ruffini G, Ibanez D, Castellano M, Dubreuil-Vall L, Soria-Frisch A, Postuma R, et al. Deep Learning With EEG Spectrograms in Rapid Eye Movement Behavior Disorder. Front Neurol. 2019;10:806. https://doi.org/10.3389/fneur.2019.00806.
Melpignano A, Parrino L, Santamaria J, Gaig C, Trippi I, Serradell M et al. Isolated rapid eye movement sleep behavior disorder and cyclic alternating pattern: is sleep microstructure a predictive parameter of neurodegeneration? Sleep. 2019;42(10). doi:https://doi.org/10.1093/sleep/zsz142.
Iranzo A, Valldeoriola F, Lomena F, Molinuevo JL, Serradell M, Salamero M, et al. Serial dopamine transporter imaging of nigrostriatal function in patients with idiopathic rapid-eye-movement sleep behaviour disorder: a prospective study. Lancet Neurol. 2011;10(9):797–805. https://doi.org/10.1016/S1474-4422(11)70152-1.
Iranzo A, Santamaria J, Valldeoriola F, Serradell M, Salamero M, Gaig C, et al. Dopamine transporter imaging deficit predicts early transition to synucleinopathy in idiopathic rapid eye movement sleep behavior disorder. Ann Neurol. 2017;82(3):419–28. https://doi.org/10.1002/ana.25026.
Iranzo A, Lomena F, Stockner H, Valldeoriola F, Vilaseca I, Salamero M, et al. Decreased striatal dopamine transporter uptake and substantia nigra hyperechogenicity as risk markers of synucleinopathy in patients with idiopathic rapid-eye-movement sleep behaviour disorder: a prospective study [corrected]. Lancet Neurol. 2010;9(11):1070–7. https://doi.org/10.1016/S1474-4422(10)70216-7.
Li Y, Kang W, Yang Q, Zhang L, Zhang L, Dong F, et al. Predictive markers for early conversion of iRBD to neurodegenerative synucleinopathy diseases. Neurology. 2017;88(16):1493–500. https://doi.org/10.1212/WNL.0000000000003838.
Dang-Vu TT, Gagnon JF, Vendette M, Soucy JP, Postuma RB, Montplaisir J. Hippocampal perfusion predicts impending neurodegeneration in REM sleep behavior disorder. Neurology. 2012;79(24):2302–6. https://doi.org/10.1212/WNL.0b013e318278b658.
Holtbernd F, Gagnon JF, Postuma RB, Ma Y, Tang CC, Feigin A, et al. Abnormal metabolic network activity in REM sleep behavior disorder. Neurology. 2014;82(7):620–7. https://doi.org/10.1212/WNL.0000000000000130.
•• Kogan RV, Janzen A, Meles SK, Sittig E, Renken RJ, Gurvits V, et al. Four-Year Follow-up of [(18) F]Fluorodeoxyglucose Positron Emission Tomography-Based Parkinson’s Disease-Related Pattern Expression in 20 Patients with Isolated Rapid Eye Movement Sleep Behavior Disorder Shows Prodromal Progression. Mov Disord. 2021;36(1):230–5. https://doi.org/10.1002/mds.28260. (This longitudinal study illustrated the presence of suprathreshold PD-related brain pattern expression, using [18F]FDG PET, in iRBD patients related to risk of phenoconversion to clinical PD.)
•• Pereira JB, Weintraub D, Chahine L, Aarsland D, Hansson O, Westman E. Cortical thinning in patients with REM sleep behavior disorder is associated with clinical progression. NPJ Parkinsons Dis. 2019;5:7. https://doi.org/10.1038/s41531-019-0079-3. (This longitudinal study showed the presence of regional cortical thinning, assessed using structural MRI, in iRBD patients at baseline was a predictor for future development of a Lewy body disease at a 3-year follow.)
Heinzel S, Berg D, Gasser T, Chen H, Yao C, Postuma RB, et al. Update of the MDS research criteria for prodromal Parkinson’s disease. Mov Disord. 2019;34(10):1464–70. https://doi.org/10.1002/mds.27802.
Lee H, Cho YW, Kim HA. The Severity and Pattern of Autonomic Dysfunction in Idiopathic Rapid Eye Movement Sleep Behavior Disorder. Mov Disord. 2015;30(13):1843–8. https://doi.org/10.1002/mds.26416.
Zitser J, During EH, Chiaro G, Miglis MG. Autonomic impairment as a potential biomarker in idiopathic REM-sleep-behavior disorder. Auton Neurosci. 2019;220: 102553. https://doi.org/10.1016/j.autneu.2019.05.005.
Alonso A, Huang X, Mosley TH, Heiss G, Chen H. Heart rate variability and the risk of Parkinson disease: The Atherosclerosis Risk in Communities study. Ann Neurol. 2015;77(5):877–83. https://doi.org/10.1002/ana.24393.
Li X, Wang K, Jia S, Zhou Z, Jin Y, Zhang X, et al. The prospective memory of patients with idiopathic REM sleep behavior disorder. Sleep Med. 2018;47:19–24. https://doi.org/10.1016/j.sleep.2018.03.019.
Terzaghi M, Zucchella C, Rustioni V, Sinforiani E, Manni R. Cognitive performances and mild cognitive impairment in idiopathic rapid eye movement sleep behavior disorder: results of a longitudinal follow-up study. Sleep. 2013;36(10):1527–32. https://doi.org/10.5665/sleep.3050.
Fantini ML, Farini E, Ortelli P, Zucconi M, Manconi M, Cappa S, et al. Longitudinal study of cognitive function in idiopathic REM sleep behavior disorder. Sleep. 2011;34(5):619–25.
Youn S, Kim T, Yoon IY, Jeong J, Kim HY, Han JW, et al. Progression of cognitive impairments in idiopathic REM sleep behaviour disorder. J Neurol Neurosurg Psychiatry. 2016;87(8):890–6. https://doi.org/10.1136/jnnp-2015-311437.
Szeto JYY, Halliday GM, Naismith SL, Lewis SJG. Exploring the Phenotype in Mild Cognitive Impairment to Aid the Prediction of Those at Risk of Transitioning to Parkinson Disease and Dementia With Lewy Bodies. J Geriatr Psychiatry Neurol. 2017;30(4):196–205. https://doi.org/10.1177/0891988717711451.
Lerche S, Machetanz G, Roeben B, Wurster I, Zimmermann M, von Thaler AK, et al. Deterioration of executive dysfunction in elderly with REM sleep behavior disorder (RBD). Neurobiol Aging. 2018;70:242–6. https://doi.org/10.1016/j.neurobiolaging.2018.06.029.
•• Arnaldi D, Chincarini A, De Carli F, Fama F, Girtler N, Brugnolo A, et al. The fate of patients with REM sleep behavior disorder and mild cognitive impairment. Sleep Med. 2021;79:205–10. https://doi.org/10.1016/j.sleep.2020.02.011. (This study shows the value of a multi-modal model, incorporating clinical and neuroimaging markers, [123I]FP-CIT DAT SPECT, to identify iRBD patients at high risk of phenoconversion within 3 years.)
Rahayel S, Postuma RB, Montplaisir J, Genier Marchand D, Escudier F, Gaubert M, et al. Cortical and subcortical gray matter bases of cognitive deficits in REM sleep behavior disorder. Neurology. 2018;90(20):e1759–70. https://doi.org/10.1212/WNL.0000000000005523.
Vendette M, Montplaisir J, Gosselin N, Soucy JP, Postuma RB, Dang-Vu TT, et al. Brain perfusion anomalies in rapid eye movement sleep behavior disorder with mild cognitive impairment. Mov Disord. 2012;27(10):1255–61. https://doi.org/10.1002/mds.25034.
Iranzo A, Isetta V, Molinuevo JL, Serradell M, Navajas D, Farre R, et al. Electroencephalographic slowing heralds mild cognitive impairment in idiopathic REM sleep behavior disorder. Sleep Med. 2010;11(6):534–9. https://doi.org/10.1016/j.sleep.2010.03.006.
Aguirre-Mardones C, Iranzo A, Vilas D, Serradell M, Gaig C, Santamaria J, et al. Prevalence and timeline of nonmotor symptoms in idiopathic rapid eye movement sleep behavior disorder. J Neurol. 2015;262(6):1568–78. https://doi.org/10.1007/s00415-015-7742-3.
Li Y, Zhang H, Mao W, Liu X, Hao S, Zhou Y, et al. Visual dysfunction in patients with idiopathic rapid eye movement sleep behavior disorder. Neurosci Lett. 2019;709: 134360. https://doi.org/10.1016/j.neulet.2019.134360.
Bertrand JA, Bedetti C, Postuma RB, Monchi O, Genier Marchand D, Jubault T, et al. Color discrimination deficits in Parkinson’s disease are related to cognitive impairment and white-matter alterations. Mov Disord. 2012;27(14):1781–8. https://doi.org/10.1002/mds.25272.
Whitfield WH, Barr GQ, Khayata MJ, Vogt PH, Keasler EM, Sanchez JM, et al. Contrast sensitivity visual acuity in REM sleep behavior disorder: a comparison with and without Parkinson disease. J Clin Sleep Med. 2020;16(3):385–8. https://doi.org/10.5664/jcsm.8212.
Cherian A, Divya KP. Genetics of Parkinson’s disease. Acta Neurol Belg. 2020;120(6):1297–305. https://doi.org/10.1007/s13760-020-01473-5.
Gamez-Valero A, Prada-Dacasa P, Santos C, Adame-Castillo C, Campdelacreu J, Rene R, et al. GBA Mutations Are Associated With Earlier Onset and Male Sex in Dementia With Lewy Bodies. Mov Disord. 2016;31(7):1066–70. https://doi.org/10.1002/mds.26593.
Sidransky E, Nalls MA, Aasly JO, Aharon-Peretz J, Annesi G, Barbosa ER, et al. Multicenter analysis of glucocerebrosidase mutations in Parkinson’s disease. N Engl J Med. 2009;361(17):1651–61. https://doi.org/10.1056/NEJMoa0901281.
Thaler A, Bregman N, Gurevich T, Shiner T, Dror Y, Zmira O, et al. Parkinson’s disease phenotype is influenced by the severity of the mutations in the GBA gene. Parkinsonism Relat Disord. 2018;55:45–9. https://doi.org/10.1016/j.parkreldis.2018.05.009.
Gan-Or Z, Mirelman A, Postuma RB, Arnulf I, Bar-Shira A, Dauvilliers Y, et al. GBA mutations are associated with Rapid Eye Movement Sleep Behavior Disorder. Ann Clin Transl Neurol. 2015;2(9):941–5. https://doi.org/10.1002/acn3.228.
Barber TR, Lawton M, Rolinski M, Evetts S, Baig F, Ruffmann C et al. Prodromal Parkinsonism and Neurodegenerative Risk Stratification in REM Sleep Behavior Disorder. Sleep. 2017;40(8). doi:https://doi.org/10.1093/sleep/zsx071.
Nalls MA, Pankratz N, Lill CM, Do CB, Hernandez DG, Saad M, et al. Large-scale meta-analysis of genome-wide association data identifies six new risk loci for Parkinson’s disease. Nat Genet. 2014;46(9):989–93. https://doi.org/10.1038/ng.3043.
Krohn L, Ozturk TN, Vanderperre B, Ouled Amar Bencheikh B, Ruskey JA, Laurent SB, et al. Genetic, Structural, and Functional Evidence Link TMEM175 to Synucleinopathies. Ann Neurol. 2020;87(1):139–53. https://doi.org/10.1002/ana.25629.
Krohn L, Wu RYJ, Heilbron K, Ruskey JA, Laurent SB, Blauwendraat C, et al. Fine-Mapping of SNCA in Rapid Eye Movement Sleep Behavior Disorder and Overt Synucleinopathies. Ann Neurol. 2020;87(4):584–98. https://doi.org/10.1002/ana.25687.
Mufti K, Yu E, Rudakou U, Krohn L, Ruskey JA, Asayesh F, et al. Novel Associations of BST1 and LAMP3 With REM Sleep Behavior Disorder. Neurology. 2021;96(10):e1402–12. https://doi.org/10.1212/WNL.0000000000011464.
Ferini-Strambi L, Fasiello E, Sforza M, Salsone M, Galbiati A. Neuropsychological, electrophysiological, and neuroimaging biomarkers for REM behavior disorder. Expert Rev Neurother. 2019;19(11):1069–87. https://doi.org/10.1080/14737175.2019.1640603.
Iranzo A, Ratti PL, Casanova-Molla J, Serradell M, Vilaseca I, Santamaria J. Excessive muscle activity increases over time in idiopathic REM sleep behavior disorder. Sleep. 2009;32(9):1149–53. https://doi.org/10.1093/sleep/32.9.1149.
Boeve BF, Silber MH, Saper CB, Ferman TJ, Dickson DW, Parisi JE, et al. Pathophysiology of REM sleep behaviour disorder and relevance to neurodegenerative disease. Brain. 2007;130(Pt 11):2770–88. https://doi.org/10.1093/brain/awm056.
Gjerstad MD, Boeve B, Wentzel-Larsen T, Aarsland D, Larsen JP. Occurrence and clinical correlates of REM sleep behaviour disorder in patients with Parkinson’s disease over time. J Neurol Neurosurg Psychiatry. 2008;79(4):387–91. https://doi.org/10.1136/jnnp.2007.116830.
Dijkstra F, Viaene M, Crosiers D, De Volder I, Cras P. Frequency and characteristic features of REM sleep without atonia. Clin Neurophysiol. 2019;130(10):1825–32. https://doi.org/10.1016/j.clinph.2019.07.018.
Eisensehr I, Linke R, Tatsch K, Kharraz B, Gildehaus JF, Wetter CT, et al. Increased muscle activity during rapid eye movement sleep correlates with decrease of striatal presynaptic dopamine transporters. IPT and IBZM SPECT imaging in subclinical and clinically manifest idiopathic REM sleep behavior disorder, Parkinson’s disease, and controls. Sleep. 2003;26(5):507–12. https://doi.org/10.1093/sleep/26.5.507.
Dijkstra F, Viaene M, De Volder I, Fransen E, Cras P, Crosiers D. Polysomnographic phenotype of isolated REM sleep without atonia. Clin Neurophysiol. 2020;131(10):2508–15. https://doi.org/10.1016/j.clinph.2020.07.005.
Fantini ML, Gagnon JF, Petit D, Rompre S, Decary A, Carrier J, et al. Slowing of electroencephalogram in rapid eye movement sleep behavior disorder. Ann Neurol. 2003;53(6):774–80. https://doi.org/10.1002/ana.10547.
Massicotte-Marquez J, Decary A, Gagnon JF, Vendette M, Mathieu A, Postuma RB, et al. Executive dysfunction and memory impairment in idiopathic REM sleep behavior disorder. Neurology. 2008;70(15):1250–7. https://doi.org/10.1212/01.wnl.0000286943.79593.a6.
Parrino L, Ferri R, Bruni O, Terzano MG. Cyclic alternating pattern (CAP): the marker of sleep instability. Sleep Med Rev. 2012;16(1):27–45. https://doi.org/10.1016/j.smrv.2011.02.003.
Terzano MG, Parrino L, Spaggiari MC. The cyclic alternating pattern sequences in the dynamic organization of sleep. Electroencephalogr Clin Neurophysiol. 1988;69(5):437–47. https://doi.org/10.1016/0013-4694(88)90066-1.
Kutlu A, Iseri P, Selekler M, Benbir G, Karadeniz D. Cyclic alternating pattern analysis in REM sleep behavior disorder. Sleep Breath. 2013;17(1):209–15. https://doi.org/10.1007/s11325-012-0675-5.
Hu Y, Yu SY, Zuo LJ, Cao CJ, Wang F, Chen ZJ, et al. Parkinson disease with REM sleep behavior disorder: features, alpha-synuclein, and inflammation. Neurology. 2015;84(9):888–94. https://doi.org/10.1212/WNL.0000000000001308.
Pagano G, De Micco R, Yousaf T, Wilson H, Chandra A, Politis M. REM behavior disorder predicts motor progression and cognitive decline in Parkinson disease. Neurology. 2018;91(10):e894–905. https://doi.org/10.1212/WNL.0000000000006134.
Ba M, Yu G, Kong M, Liang H, Yu L. CSF Abeta1-42 level is associated with cognitive decline in early Parkinson’s disease with rapid eye movement sleep behavior disorder. Transl Neurodegener. 2018;7:22. https://doi.org/10.1186/s40035-018-0129-5.
Zhang WJ, Shang XL, Peng J, Zhou MH, Sun WJ. Expression of prion protein in the cerebrospinal fluid of patients with Parkinson's disease complicated with rapid eye movement sleep behavior disorder. Genet Mol Res. 2017;16(1). doi:https://doi.org/10.4238/gmr16019022.
Politis M. Neuroimaging in Parkinson disease: from research setting to clinical practice. Nat Rev Neurol. 2014;10(12):708–22. https://doi.org/10.1038/nrneurol.2014.205.
Wu P, Yu H, Peng S, Dauvilliers Y, Wang J, Ge J, et al. Consistent abnormalities in metabolic network activity in idiopathic rapid eye movement sleep behaviour disorder. Brain. 2014;137(Pt 12):3122–8. https://doi.org/10.1093/brain/awu290.
Meles SK, Renken RJ, Janzen A, Vadasz D, Pagani M, Arnaldi D, et al. The Metabolic Pattern of Idiopathic REM Sleep Behavior Disorder Reflects Early-Stage Parkinson Disease. J Nucl Med. 2018;59(9):1437–44. https://doi.org/10.2967/jnumed.117.202242.
Huang Z, Jiang C, Li L, Xu Q, Ge J, Li M, et al. Correlations between dopaminergic dysfunction and abnormal metabolic network activity in REM sleep behavior disorder. J Cereb Blood Flow Metab. 2020;40(3):552–62. https://doi.org/10.1177/0271678X19828916.
Meles SK, Vadasz D, Renken RJ, Sittig-Wiegand E, Mayer G, Depboylu C, et al. FDG PET, dopamine transporter SPECT, and olfaction: Combining biomarkers in REM sleep behavior disorder. Mov Disord. 2017;32(10):1482–6. https://doi.org/10.1002/mds.27094.
Miyamoto T, Miyamoto M, Inoue Y, Usui Y, Suzuki K, Hirata K. Reduced cardiac 123I-MIBG scintigraphy in idiopathic REM sleep behavior disorder. Neurology. 2006;67(12):2236–8. https://doi.org/10.1212/01.wnl.0000249313.25627.2e.
Miyamoto T, Miyamoto M, Suzuki K, Nishibayashi M, Iwanami M, Hirata K. 123I-MIBG cardiac scintigraphy provides clues to the underlying neurodegenerative disorder in idiopathic REM sleep behavior disorder. Sleep. 2008;31(5):717–23. https://doi.org/10.1093/sleep/31.5.717.
Knudsen K, Fedorova TD, Hansen AK, Sommerauer M, Otto M, Svendsen KB, et al. In-vivo staging of pathology in REM sleep behaviour disorder: a multimodality imaging case-control study. Lancet Neurol. 2018;17(7):618–28. https://doi.org/10.1016/S1474-4422(18)30162-5.
Miyamoto T, Miyamoto M, Iwanami M, Hirata K. Follow-up study of cardiac (1)(2)(3)I-MIBG scintigraphy in idiopathic REM sleep behavior disorder. Eur J Neurol. 2011;18(10):1275–8. https://doi.org/10.1111/j.1468-1331.2011.03392.x.
Campabadal A, Segura B, Junque C, Iranzo A. Structural and functional magnetic resonance imaging in isolated REM sleep behavior disorder: A systematic review of studies using neuroimaging software. Sleep Med Rev. 2021;59: 101495. https://doi.org/10.1016/j.smrv.2021.101495.
• Pena-Nogales O, Ellmore TM, de Luis-Garcia R, Suescun J, Schiess MC, Giancardo L. Longitudinal Connectomes as a Candidate Progression Marker for Prodromal Parkinson’s Disease. Front Neurosci. 2018;12:967. https://doi.org/10.3389/fnins.2018.00967. (Using a machine learning algorithm, this study highlights that a longitudinal brain connectome progression score, determined using connectivity data from diffusion MRI, could discriminate progression in RBD patients.)
Griffanti L, Klein JC, Szewczyk-Krolikowski K, Menke RAL, Rolinski M, Barber TR, et al. Cohort profile: the Oxford Parkinson’s Disease Centre Discovery Cohort MRI substudy (OPDC-MRI). BMJ Open. 2020;10(8): e034110. https://doi.org/10.1136/bmjopen-2019-034110.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights
All reported studies/experiments with human or animal subjects performed by the authors have been previously published and complied with all applicable ethical standards (including the Helsinki Declaration and its amendments, institutional/national research committee standards, and international/national/institutional guidelines).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Sleep
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
de Natale, E.R., Wilson, H. & Politis, M. Predictors of RBD progression and conversion to synucleinopathies. Curr Neurol Neurosci Rep 22, 93–104 (2022). https://doi.org/10.1007/s11910-022-01171-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11910-022-01171-0