Abstract
End-organ failure is associated with high mortality and morbidity, in addition to increased health care costs. Organ transplantation is the only definitive treatment that can improve survival and quality of life in such patients; however, due to the persistent mismatch between organ supply and demand, waiting lists continue to grow across the world. Careful intensive care management of the potential organ donor with goal-directed therapy has the potential to optimize organ function and improve donation yield.
Similar content being viewed by others
Introduction
Every 10 min, one person is added to the US national transplant waiting list, and while waiting, approximately 22 people die each day [1]. Organ donation (OD) is a selfless deed with the potential to reduce health care costs associated with organ support to patients with end-organ failure, and make a significant impact on society by improving the survival and quality of life of recipients [2, 3].
The neuroscience intensive care unit frequently harbors patients with devastating neurologic illnesses relying on advanced organ support. Not uncommonly, end-of-life decision-making following neuroprognostication results in withdrawal of life-sustaining therapies (WLST) for patients in whom potential for recovery is deemed dismal. Moreover, further irreversible neurologic damage leading to brain death (BD) happens not infrequently. As a result, the ICU staff is commonly involved in the care of a potential organ donor and has a key role in preserving the option of OD. Major steps in this process include prompt notification of the organ procurement organization (OPO) following the identification of a potential donor and implementation of donor goal-directed therapies. In addition, the mission of fostering the adequate environment for grieving families while the dignity of the deceased person is maintained throughout the process of donation is a responsibility of ICU staff. This is important, as family dissatisfaction with care provided during a hospitalization that involved OD has been associated with further complicated adaptation to the loss of a loved one [4, 5]. In the past, the role of the intensivist often ended upon contacting the OPO and determining the death of the potential donor. However, accumulating evidence advocates for continued involvement of the intensivist providing intensive care and organ support to the potential organ donor, as it may maximize the yield of retrieved organs by systematically meeting donor management goals [6–12]. This review will focus on the intensive care management of the potential organ donor where the intensivist has a pivotal role in contributing to narrowing the gap between organ supply and demand.
Background of Deceased Organ Donation
Deceased donors have been the main source of solid organs for transplantation [13], and all organs from deceased donors were initially harvested from patients soon after cardiorespiratory arrest. The practice of donation after cardiorespiratory death (DCD) was exclusive until the concept of BD became universally accepted by the medical community [14] and sealed by the Uniform Determination of Death Act (UDDA) in 1980 [15, 16]. The Uniform Anatomical Gift Act then established the “Dead Donor Rule” (DDR), mandating that organ harvesting must follow the determination of the donor’s death; thus, removal of organs must not lead to one’s death [17]. Despite these delineations, pronouncing death can be quite challenging without precise guidelines with a stepwise approach, and the potential for questioning the legitimacy of death determination and, ultimately donation, arises [18•]. To address this issue, the World Health Organization joined Health Canada and the Canadian Blood Services to develop an international guideline for the definition of death [18•]; the product of this effort is the following definition:
“Death is the permanent loss of capacity for consciousness and all brainstem functions. This may result from permanent cessation of circulation or catastrophic brain injury.”
This definition is compliant with the irreversibility criteria, a requirement by the UDDA, by implying that the permanence of the circulatory arrest exists by withholding resuscitation efforts once loss of circulatory function is established [19]. However, specific nuances to the practical determination of death are still left to the discretion of institutional protocols.
Donation After Circulatory Determination of Death or Non-heart-beating Donors
The need to expand the donor pool has fueled further the development of new DCD programs. Currently, DCD remains an important source of solid organs around the world, as specific cultural norms may challenge the recognition and acceptance of the BD concept, thus limiting the number of DBD donors. DCD accounts for more than 20 % of all deceased organ donation worldwide [20], and over 60 % in Japan [21]. In the USA, approximately 10 % of all organ transplantations are from DCD [22].
DCD donors can be categorized according to the updated Maastricht Classification (Table 1) into five different categories, each having their own challenges with minimization of organ damage after death determination, fostering organ preservation and viability, and their ethical aspects [23, 24].
Death Determination
Death by circulatory criteria must be irreversible, and any subsequent restoration of spontaneous circulation, either spontaneously or as a result of an intervention, precludes death determination. In clinical practice, the absence of a pulse, respiratory incursions, and a heart beat are the cardinal signs in the evaluation for death of patient. Often, a heart monitor displaying the cardiac electrical function is not available or required. However, in the DCD context, the documentation of cessation of cardiac electric function (electrical asystole) is required in some institutions in addition to lack of cardiac contractility (mechanical asystole). The minimum set of criteria agreed upon by the forum deriving the international guidelines for death determination in DCD include absent palpable pulse, breath sounds, heart sounds, respiratory effort, and loss of pulsatile arterial blood pressure along with coma with fixed and dilated pupils [18•]. Notably, electrical asystole was not a requirement; however, it may be left to the discretion of each institution [25]. In addition, pulseless apnea must be observed for a few minutes while resuscitation measures are withheld in order to document the irreversibility of absence of spontaneous forward circulation. The observation time is necessary, as in rare occasions forward circulation is achieved spontaneously, a phenomenon called autoresuscitation. The minimal duration of observation varies according to institutional guidelines, but usually ranges from 2 to 5 min [26]. Observation of pulseless apnea for 2 min may be the minimum time necessary, as this phenomenon is exceedingly rare in the absence of prior cardiopulmonary resuscitation (CPR) [27].
Identification of cDCD Candidates
Usually, to allow for a successful DCD, the allotted time from WLST to death should be optimally less than 60 min and certainly no more than 120 min [28]. This is the most important variable that will determine whether the patient can become an organ donor, as it impacts the viability of organs. Predicting the time of death from the time of WLST can be challenging, and multiple prediction models have been proposed [29–33, 34•]:
-
The University of Wisconsin DCD Evaluation Tool is able to accurately predict suitability for organ donation in 83.7 % of cases [29]. It is based on a scoring system that stratifies patients into high, moderate, and low risk groups for maintaining spontaneous breathing once ventilatory support is removed. The variables that are accounted for include vasopressor requirements, body mass index (BMI) and respiratory performance during a 10-min interval from the discontinuation of ventilatory support.
-
The United Network for Organ Sharing criteria was associated with a positive predictive value for suitability for donation of 63 % [30]. The cumulative presence of more than one criterion based on respiratory parameters and hemodynamic support requirements increases the odds of death within 60 min of WLST.
-
The DCD-N score had a good performance for predicting death within 60 min (area under ROC curve of 0.81) [32] and was the first to include markers of severe neurologic dysfunction. Points are attributed to elements constituting a poor neurologic exam—absence of corneal and cough reflexes, no motor response or presence of extensor posturing—in addition to a high oxygenation index.
-
The nomogram for time of death prediction following WLST in neurologically devastated patients was recently developed as an alternative tool. By using a nomogram with a graphical representation of the numerical probability of a clinical event based on the statistical predictive model, this became an attractive tool. This system includes a comprehensive list of radiologic and clinical neurologic variables with their respective weighted scores, and the total sum is plotted in the nomogram, where probabilities for 30-, 60-, 120-, and 240-min mortalities are given [34•]. The ability to predict organ suitability based on different mortality times is promising, and further validation of this nomogram in a large, multicenter cohort is underway [35].
Pathophysiology of Organ Injury
The time interval during which an organ is vulnerable to an ischemic insult is known as warm ischemia time (WIT) and includes the time when there is blood flow but insufficient circulation to oxygenate blood and deliver it to the organs and tissues [36]. The total WIT is considered the interval from removal of artificial ventilation to initiation of organ perfusion, [37] and the functional ischemia time (or true WIT) is the period from start of hypotension and/or severe hypoxemia (both of which with variable thresholds) to initiation of organ perfusion [37–39]. Rates of graft complications are directly proportionate to WIT, and tolerance to ischemia is organ-specific; thus, particular attention to the duration of WIT is important when assessing organ suitability for donation [28, 40–43]. The time interval from organ removal until transplantation with restoration of circulation regardless of in situ preservation is called cold ischemia time (CIT), [36] and also has a significant impact in graft function and survival.
The pathophysiology of dying involves complex mechanisms that play an important role in the overall organ status. There are several types of organ-specific dysfunction post-transplantation, invariably related to ischemic times and reperfusion injury. Definitions vary across studies, making comparison of incidence rates and risk factors challenging.
Organ Support
The intensive care management of the potential DCD donor is comprised of several interventions to optimize organ viability, and some of them may occur prior to, during, or after death. Figure 1 summarizes the key points in the clinical pathway for cDCD based on practice guidelines derived by the American Society of Transplant Surgeons (ASTS) [37]. These guidelines provide recommendations regarding each stage of the process, including the development of local protocols, the approach used with families and surrogates when obtaining informed consent, limitations of specific roles by transplant team personnel, WLST, death determination, maintenance of an adequate grieving environment, organ procurement and ex vivo organ perfusion techniques, and organ-specific particulars [37]. The consent for DCD should include the possibility of patient survival despite WLST, which would imply the inability to provide the intended organs [14, 26, 28, 36]. In order to minimize WIT and streamline the process of organ harvesting, WLST should take place in the operating room whenever possible. Families should be respected and given the opportunity to spend time with the patient as much as possible, particularly in the period from WLST to pulseless apnea is noted [26]. The use of comfort measures is allowed, even if the unintended consequence is the potential hastening of death (commonly referred to as the rule of double effect); however, the practitioner providing end-of-life care should not be involved in the procurement process [28].
Clinical pathway for cDCD. This flowchart was created based on the work from multiple sources [37, 41, 105–111]. The most commonly used cold storage solution for the majority of organs is the University of Wisconsin (UW) solution (gold standard) [80]. Anti-ischemic drugs, hormones, proteasome inhibitors, carbonic anhydrase II, statins, and anti-inflammatory agents are being studied as potential additives to preservative solutions [109]. a During this phase, heparinization and thrombolysis are employed, and there is a consideration of hemodilution and leukofiltration techniques. Machine perfusion of the kidneys can be pulsatile or continuous, and normothermic or hypothermic. b Machine perfusion in pancreas transplantation is currently an investigational procedure; it can be pulsatile or continuous, and normothermic or hypothermic. The delicate structure of the pancreas, particularly its endothelium, challenges this technique, requiring strict pressure and flow limits. Normothermic perfusion allows assessment of exocrine and endocrine responses in pancreas grafts [107], and viability assessment in liver grafts [109]. c Machine perfusion in lungs have additional organ-specific advantages, including allowing for the administration of antimicrobial therapy, thrombolytics, and gene therapies [20]. Additionally, it allows for organ function assessments prior to transplantation. Normothermic lung perfusion is preferred. CPAP continuous positive airway pressure, DCD donation after cardiorespiratory death, IVF intravenous fluid, NS normal saline, OR operating room, WLST withdrawal of life-sustaining therapy
Specific pharmacologic interventions promoting optimal organ perfusion to counteract the microcirculatory failure associated with ischemic-reperfusion injury such as vasodilators (e.g., phentolamine), inotropes, or heparinoids remain controversial, since their administration is antemortem and their sole purpose is to benefit the potential recipient [44]. Further controversies afflict practices of antemortem administration of thrombolytics and femoral cannulation in preparation for cold preservation solution administration. Local practices should follow specific legislation as well as institutional policies and should only be employed after specific consent is obtained.
The use of mechanical or manual chest compressions following cessation of circulation for organ support is not universally recommended despite being widely used. This recommendation is due to the increased risk of achieving return of spontaneous circulation (ROSC), a concern which challenges the irreversibility of the circulatory arrest [45]. In addition, both extracorporeal membrane oxygenation (ECMO) and mechanical chest compressions whenever used as a method of organ preservation should be preceded by a careful explanation to donor families of the possibility of restoration of brain perfusion before irreversible cessation of all functions or ROSC [19, 46].
Ethical Considerations
Organ donation in DCD may raise ethical questions related to the determination and timing of death, timing and type of interventions promoting organ support, possible conflicts of interest in selected team members participating in different stages of the donation process, methods of obtaining consent, and adequacy of the environment and atmosphere for grieving families when saying goodbye to their loved ones [14]. In addition, in countries where euthanasia is legal and morally acceptable, organ harvesting preceding medically assisted suicide defies the death donor rule and stirs further debate [47].
Future Trends
DCD has a critical role in filling the gap between organ demand and supply. The expansion of programs may rely on further developments on the acceptable boundaries of DCD while maintaining legal standards for death determination and on the creation of a uniform medical standard with a stepwise approach to death determination. In addition, harnessing predictive models for timing of death from WLST may optimize logistics and resources utilization. Advances in organ support and preservation techniques are needed to continue to push the limits of WIT, and consequently may increase the pool of harvested organs. Finally, further public and political discussions are needed regarding acceptable strategies to include death by OD as a possible amendment to the DDR, should society agree that this practice is morally acceptable.
Donation After Brain Determination of Death, or Heart-Beating Donors
Advances in resuscitation over half a century ago, specifically positive pressure mechanical ventilation and alternating current cardiac defibrillation, allowed for a new neurological state to be recognized: the irretrievable coma [48]. Patients without evidence of central nervous system (CNS) activity, completely unresponsive to any stimulation, and devoid of all brainstem functions were now able to have their organs perfused with oxygenated blood, sometimes for long periods of time, by means of artificial support. The concept of BD [49] arose to address the rising need to modify the death definition in order to provide closure to families and prioritize the use of scarce medical resources when cessation of all brain function occurs. Contemporarily, advances in organ transplantation techniques leading to successful outcomes fomented the need for a modern definition of death, congruent to the medical developments of the time. From 1968 to 2010, the scientific community developed and honed the definition of BD, including the derivation of a set of criteria guiding clinicians with updated practice parameters for its determination by the American Academy of Neurology (AAN) [50–52]. This document includes a summary of available evidence and provides a stepwise approach to BD determination including checklists, prerequisites, clinical and ancillary testing as well as important details pertinent to BD documentation [52]. Donation following BD swiftly became the preferred method of organ harvesting in the places where BD is culturally and legally accepted, as DBD provides a higher yield and improved viability of harvested organs when compared to DCD [53]. In fact, it is the ASTS recommendation to pursue DBD over DCD in situations where the potential organ donor is or will likely become brain dead, as long as the family is in agreement [37]. The prompt referral of patients at risk for “imminent neurological death” by the treating team is of major importance. Other proposed triggers used in clinical practice to identify such patients include the Organ Procurement and Transplantation Network (OPTN) criteria (age ≤70 years old, mechanically ventilated patients with absence of ≥3 brainstem reflexes as a result of a severe neurologic injury) [54], a Glasgow Coma Score of 3 plus the absence of ≥3 brainstem reflexes, and a Full Outline of Unresponsiveness score (FOUR score) of 0 [55].
Death Determination
The diagnosis of BD is based on clinical findings and is supported by ancillary testing in situations where the complete clinical examination is not possible or not entirely reliable. A comprehensive checklist for BD determination includes the cardinal rules of knowing the cause of the neurological state and its irreversibility. This is supported by neuroimaging, and there must be an apneic unresponsive state with brainstem, areflexia, and the exclusion of potential confounders [56]. For a summary of the evolution of BD definitions and for a checklist guiding the determination of BD including a stepwise approach for standard apnea testing, we recommend the review paper by Hwang et al. [57].
The technique for standard apnea testing has been associated with several potential clinical complications, which include arterial hypotension, acidosis, hypoxemia, pneumothorax, pneumomediastinum, bradycardia, atrial fibrillation, myocardial infarction, and cardiac arrest [58]. Hypotension may be due to auto-positive end-expiratory pressure (PEEP), but frequently it is related to ensuing severe respiratory acidosis; thus, transient hyperventilation when apnea testing is completed is recommended. In addition, making sure the patient is euvolemic prior to performing apnea testing is of great importance to avoid test abortion due to hypotension. The occurrence of pneumothorax and pneumomediastinum reflects the potential for barotrauma due to air trapping and auto-PEEP when the standard 6 mm tubing to deliver continuous supplemental oxygen is used. The use of pressure tubing with an outside diameter of 3 mm instead has been shown to reduce the potential for this complication [59], particularly in patients with endotracheal tubes ≤7.0 size. The prolonged apnea interval is associated with atelectasis and decreased PaO2/FiO2 ratios despite adequate pre-oxygenation, which may impair the respiratory function of the potential lung donor and organ suitability for donation [60]. A single recruitment maneuver following apnea testing by using a PEEP of 35 cm H2O for 40 s (or equivalent) has been shown to prevent such a decrease in PaO2/FiO2 ratios [60]. Of importance, prior to any recruitment maneuver, the clinician must be certain of the patient’s euvolemia, as the consequent decrease in venous return, although transient, may lead to profound hypotension. In our experience, aiming for a systolic blood pressure of ≥140 or a mean arterial pressure ≥90 mmHg with the use of vasopressors prevents any clinically significant hypotension during the recruitment maneuver. Alternatively, performing apnea testing with continuous positive airway pressure (CPAP) is also promising in avoiding de-recruitment and can be used in different ways: directly by the ventilator, by means of a CPAP valve with a reservoir (AMBU TM), or through a T-piece system [61]. Finally, the use of the Boussignac CPAP system is another innovative technique that allows patients with severe respiratory disturbances to successfully complete apnea testing who would otherwise require ancillary testing for BD diagnosis [62]. There is now evidence that patients being supported by ECMO can safely undergo apnea testing by CPAP [63].
Pathophysiology of Systemic Complications in Brain Death
Ischemia-reperfusion injury is inherent to the process of organ transplantation and may culminate in rejection and graft failure. The mechanisms implicated in this pattern of organ injury are intimately related to ischemia times and the systemic inflammatory process driven by reactive oxygen species. In addition, severe brain injury also results in further systemic pro-inflammatory responses, leading to leukocyte recruitment to major organs, generation of reactive oxygen species, release of inflammatory mediators, increased vasculature permeability, and ultimately organ dysfunction [64, 65]. Inflammatory mediators in the bowel and spleen are then activated through increased vagal input [66] and expose the patient to endotoxins which exacerbate pulmonary inflammation, resulting in neurogenic pulmonary edema (NPE) [67, 68]. In addition, the presence of blood brain barrier disruption and the resulting bidirectional access of local mediators from the brain to the blood, and vice versa, further exacerbates this inflammatory cascade and results in additional organ injury [69]. The massive release of catecholamines reflects the attempt to enhance brain perfusion to overcome the increased intracranial pressure. The ensuing dysautonomia is due to unopposed sympathetic input from eventual loss of the vagal parasympathetic nucleus [65, 70]. However, this has catastrophic systemic effects: end-organ vasoconstriction and tissue hypoperfusion; increased ino/chronotropy, demand ischemia, arrhythmias, cardiac cellular death, and reduced cardiac output; capillary leakage, pulmonary vasoconstriction, and impaired oxygenation [65, 71]. These patients have a higher end-diastolic volume index, lower end-systolic pressure-volume relationship, reduced contractility, and an elevated end-diastolic pressure-volume relationship, all signifying impaired systolic and diastolic function [72]. At later stages of progressive brain injury, hypotension ensues due to a combination of loss of sympathetic drive, concurrent hormonal failure (e.g., relative hypothyroid state, adrenal insufficiency, and diabetes insipidus), and metabolic acidosis from the shifting of aerobic to anaerobic metabolism [20, 65, 73]. The release of tissue thromboplastin by the ischemic brain promotes activation of the coagulation cascade which, coupled with endothelial disruption, may lead to disseminated intravascular coagulation (DIC) [74]. The endothelial injury and microthrombi formation exacerbates the microcirculatory failure with further ischemic injury [75].
As neuronal cell death progresses in a cranial-caudal manner, there is concurrent failure of the hypothalamic-hypophyseal axis, leading to severe endocrinopathy that varies in timing and severity. An early sign of endocrine failure is diabetes insipidus from depletion of anti-diuretic hormone (ADH), resulting in massive diuresis, hyperosmolality, hypernatremia, and volume depletion. This is usually followed by decreased thyroid-stimulating hormone (TSH) secretion which, in addition to decreased peripheral conversion of tetraiodothyronine (T4), results in a rapid decline in free triiodothyronine (T3) [76]. The thyroid failure, in turn, allows for the depletion of high-energy phosphates and impaired cardiac contractility, the shift to anaerobic metabolism and resulting increase in lactate [74]. The lactic acidosis is potentiated by the decrease in available intracellular glucose from lower circulating insulin levels. Hyperglycemia is further exacerbated by the catecholamine storm and promotes further hypovolemia due to osmotic diuresis. Finally, donor stress responses are blunted due to adrenal insufficiency often seen in BD, and decreased levels of cortisol and adrenocorticotropic hormone (ACTH) contribute to hypotension and cardiovascular instability in these patients [76]. Table 2 provides an overview of the prevalence of systemic complications in BD.
Organ Support
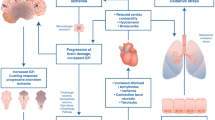
Organ preservation initiates with the optimal management of the potential organ donor, continues during procurement and storage, and targets increasing the likelihood of procurement in potential donors, the yield of transplantable organs per donor, and improving graft function after transplantation [77]. Notably, longer duration of a donor’s BD does not seem to be detrimental to renal grafts, although it was thought to do so in the past [78]. Nevertheless, the BD interval does correlate with recipient mortality in heart transplants; no data has been provided regarding optimization of donor management during this interval [79]. Controversy remains regarding the ideal time from diagnosis to procurement in DBD. Accumulating evidence suggests that taking the time to stabilize the patient and institute measures to optimize organ function prior to organ retrieval may be better than rushing at the peak of an inflammatory and sympathetic storm [78]. The cascade of multisystem changes resulting from BD warrants a systematic approach to the potential DBD donor. Figure 2 provides a flowchart with the key points in the clinical pathway for DBD.
Clinical pathway and algorithm for the potential DBD organ donor management. This flowchart was created based on multiple sources [20, 60, 80, 84••, 88, 89, 111]. Organ preservation techniques are similar to the ones demonstrated in the DCD flowchart. CPAP—continuous positive airway pressure. CVP—central venous pressure. DI—diabetes insipidus. DIC—disseminated intravascular coagulation. DOB—dobutamine. DOPA—dopamine. DVT—deep venous thrombosis. EVLW–extravascular lung water. EPI—epinephrine. GFS—glucose fingerstick. HOB—head of bed. HR—heart rate. IV—intravenous. K—potassium. LVEF—left ventricular ejection fraction. MAP—mean arterial pressure. Mg—magnesium. Na—sodium. NE—norepinephrine. P—phosphorous. PCWP—pulmonary capillary wedge pressure. PEEP—positive end expiratory pressure. OPO—organ procurement organization. RM—recruitment maneuver. SG—specific gravity. SVR—systemic vascular resistance. SVT—supraventricular tachycardia. VSP—vasopressin. VT—ventricular tachycardia. TV—tidal volume. Temp—temperature. UOP—urinary output
Endocrine Support
The collapse of hypothalamic-pituitary axis and resulting hormonal failure may lead to detrimental effects at molecular, cellular, and tissue levels. Hormonal support is widely used in this scenario; however, its efficacy has not been completely elucidated [77]. Promising results regarding improvement in hemodynamics, procurement yield, and post-transplant cardiac graft function with the use of thyroid hormones conflict with the results of a meta-analysis of randomized prospective studies which failed to support these effects [80, 81]. Hormonal replacement is indicated when hemodynamic goals are not met with initial fluid resuscitation and the vasopressor needs escalate above the desired doses (dopamine or dobutamine >10 mcg/kg/min, or >0.05 mcg/kg/min for epinephrine or norepinephrine) or left ventricle ejection fraction (LVEF) remains <45 %. Regimens used across the world vary widely, and oral and intravenous formulations seem equivalent [82]. In addition, steroid support has been shown to improve oxygenation and the need for vasopressors, which can increase rates of high-yield procurement [83]; however, the ideal dosing remains to be clarified in further studies. Vasopressin use has been shown to increase procurement yield and is helpful in reducing inotrope use [80], in addition to its value in the treatment of diabetes insipidus and lowering sodium levels. Maintaining a target sodium of <155 mEq/L is recommended due to poor liver graft survival with higher levels [84••]. The use of a combination of thyroid hormone, corticosteroid, anti-diuretic hormone, and insulin seems to be the best approach for multiple organ procurement [85].
Hemodynamic Support
The assessment of the potential BD organ donor is challenging. Frequently, coexisting comorbidities such as NPE, distributive shock, diabetes insipidus, dysautonomia, and hormonal failure may cloud the interpretation of hemodynamic parameters and urinary output, which are cardinal signs used in critical care to guide this assessment. In addition, patients with severe neurologic injury preceding BD were often given osmotherapy, which may also affect the volume status and lead to metabolic derangements that are hard to interpret. The intensivist is encouraged to use whatever tools are available at the bedside to guide fluid therapy, such as transthoracic echocardiography, lithium dilution or thermodilution approaches [86]. Notably, the MOnIToR trial, the first large, multicenter, randomized study using a protocol-guided fluid therapy targeting cardiac index, MAP, and pulse pressure variation using LiDCOTM, failed to show a higher yield of harvesting with such an approach [87]. Further studies using simplified and easily accessible devices may be helpful in the future. Overall goals to be achieved in the management of the potential DBD donor are maintaining euvolemia and optimal perfusion pressure (MAP ≥60–70 mmHg), target urine output of at least 1 mL/kg/h, and LVEF of at least 45 % [84••] while utilizing the lowest possible rates of vasopressor support. Serial lactate levels, mixed venous saturation values, arterial blood gases, and invasive or noninvasive hemodynamic measurements—like central venous pressure, pulmonary capillary wedge pressure, cardiac index, and extravascular lung water—are helpful to guide further therapy. If vasopressors are needed, consideration of dopamine and vasopressin as first-line agents is recommended; in severe shock, norepinephrine, phenylephrine, dobutamine, and epinephrine are acceptable choices. Arrhythmias are frequent in BD donors and may be difficult to treat, resulting in further hemodynamic instability. Avoiding overreaction to catecholamine storm-related arrhythmias is key; thus, short-acting agents are preferred [84••]. Bradyarrhythmias are better treated with isoproterenol or epinephrine, since they may be refractory to atropine due to break down of the vagal nucleus [88]. Ventricular tachyarrhythmias should be treated with lidocaine or amiodarone; the latter is also the first choice for supraventricular tachyarrhythmias [88].
In potential renal donors, the consideration of low dose dopamine infusion (4 mcg/kg/min) is warranted as it has been shown to reduce the need for dialysis post-transplantation without increasing side effects [89] due to possible attenuation of ischemic-reperfusion injury by stimulation of D3 receptors [90].
Ventilatory Support
The lungs of DBD donors are susceptible to the development of primary graft dysfunction due to the presence of a systemic inflammatory response and ensuing NPE, an increased risk of aspiration, as well as their vulnerability to ventilator-associated lung injury [20]. The implementation of a lung donor management protocol consisting of lung protective ventilation (6–8 mL/kg tidal volumes and higher PEEP), apnea testing with CPAP, optimal positioning of the patient, optimal volume status, and frequent recruitment maneuvers for potential donors who did not meet the PaO2/FiO2 threshold for donation have been associated with doubled rates of lung donation without an increase in graft dysfunction [91, 92]. Despite prior concerns of a restrictive fluid balance leading to a negative impact on kidney grafts, maintaining goal central venous pressures <8 mmHg seems safe [20, 93, 94]. Inhaled nitric oxide should be considered in BD donors with NPE to improve pulmonary perfusion by causing local vasodilatation and decreased vascular resistance [95, 96]. There is no role for β-agonist inhalers in such cases [97].
Nutritional Support
Enteric nutrition should be continued unless there is a contraindication, as it increases glycogen supply and has the potential to optimize allograft function [84••].
Ethical Considerations
Conflicts between families’ inclinations and the patient’s prior wishes may occur and should be addressed on a case-by-case basis by a collaboration of OPO staff and leadership, healthcare providers, hospital administration, and the patient’s family [84••]. An ethics consult may also be beneficial if conflict continues. Explaining to families the potential donor’s prior designation as a donor is of particular importance in solving this conflict. In addition, some families may not accept the irreversibility of a BD diagnosis. A careful explanation by the intensivist of the meaning of BD is of utmost importance, as some families may have difficulty understanding death despite a beating heart of their loved one [98, 99].
Future Trends
The standardization of clinical assessment and ancillary testing in BD determination would facilitate a uniform practice and further development of DBD programs worldwide. In addition, the development of new methods of apnea testing targeting improved safety would expand its use and avoid the need of ancillary testing and further delays in BD diagnosis. Recently, the use of mild hypothermia to 34–35 °C in potential kidney donors was associated with decreased delayed graft function among recipients, without an increase in adverse events [100]. Further studies investigating the optimal target temperature in potential organ donors are warranted. Other aspects of the critical care management of the potential BD donor that require further studies include exploration of ideal hormonal support regimens, optimal fluid management protocols, and discovery of potential inhibitors of the BD-induced inflammatory response.
Conclusion
Significant variability in the intensive care management of the potential organ donor exists across the world. The development of international standards for deceased OD practices targeting improved regulation, optimal procurement, and improved yields are needed. Recognizing the positive impact of the intensivist’s role in increasing OD yields by streamlining the care of the potential organ donors is a promising measure to help decrease the gap between organ supply and demand.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
OPTN/UNOS. The Organ Procurement and Transplantation Network Data. 2015 [June 6, 2016]; Available from: http://optn.transplant.hrsa.gov. .
Wolfe RA et al. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med. 1999;341(23):1725–30.
Pinson CW et al. Health-related quality of life after different types of solid organ transplantation. Ann Surg. 2000;232(4):597–607.
Cleiren MP, Van Zoelen AA. Post-mortem organ donation and grief: a study of consent, refusal and well-being in bereavement. Death Stud. 2002;26(10):837–49.
Merchant SJ et al. Exploring the psychological effects of deceased organ donation on the families of the organ donors. Clin Transplant. 2008;22(3):341–7.
Angel LFLF. Impact of a lung transplantation donor-management protocol on lung donation and recipient outcomes. Am J Respir Crit Care Med. 2006;174(6):710–6.
Salim AA. The effect of a protocol of aggressive donor management: implications for the national organ donor shortage. J Trauma: Inj Infect Crit Care. 2006;61(2):429–35.
Singbartl KK. Intensivist-led management of brain-dead donors is associated with an increase in organ recovery for transplantation. Am J Transplant. 2011;11(7):1517–21.
Malinoski DJ et al. The impact of meeting donor management goals on the number of organs transplanted per donor: results from the United Network for Organ Sharing Region 5 prospective donor management goals study. Crit Care Med. 2012;40(10):2773–80.
Callahan DS et al. Trends in organ donor management: 2002 to 2012. J Am Coll Surg. 2014;219(4):752–6.
Abuanzeh RR. Early donor management increases the retrieval rate of hearts for transplantation in marginal donors. Eur J Cardiothorac Surg. 2015;47(1):72–7.
Patel MS et al. The impact of meeting donor management goals on the number of organs transplanted per expanded criteria donor: a prospective study from the UNOS Region 5 Donor Management Goals Workgroup. JAMA Surg. 2014;149(9):969–75.
Healthcare, E.D.f.t.Q.o.M. International figures on donation and transplantation 2014. 2014.
E. Committee. Recommendations for nonheartbeating organ donation. A position paper by the Ethics Committee, American College of Critical Care Medicine, Society of Critical Care Medicine. Crit Care Med. 2001;29(9):1826–31.
NCCUSL, T.N.C.o.C.o.U.S.L. Uniform Law Commission: determination of death act summary. 1980 [November 10, 2015]; Available from: http://www.uniformlaws.org/ActSummary.aspx?title=Determination%20of%20Death%20Act.
M. Consultants. Guidelines for the determination of death. Report of the medical consultants on the diagnosis of death to the President’s Commission for the Study of Ethical Problems in Medicine and Biomedical and Behavioral Research. JAMA. 1981;246(19):2184–6.
Robertson JAJ. The dead donor rule. Hast Cent Rep. 1999;29(6):6.
Shemie SD et al. International guideline development for the determination of death. Intensive Care Med. 2014;40(6):788–97. Of importance: First international initiative deriving international standards for death determination.
Bernat JLJL. The circulatory-respiratory determination of death in organ donation. Crit Care Med. 2010;38(3):963–70.
Munshi L, Keshavjee S, Cypel M. Donor management and lung preservation for lung transplantation. Lancet Respir Med. 2013;1(4):318–28.
Bendorf A et al. An international comparison of the effect of policy shifts to organ donation following cardiocirculatory death (DCD) on donation rates after brain death (DBD) and transplantation rates. PLoS One. 2013;8(5), e62010.
Blackstock MJ, Ray DC. Organ donation after circulatory death: an update. Eur J Emerg Med. 2014;21(5):324–9.
Kootstra GG. Categories of non-heart-beating donors. Transplant Proc. 1995;27(5):2893.
Ridley SS. UK guidance for non-heart-beating donation. Br J Anaesth. 2005;95(5):592.
Fugate JE et al. Variability in donation after cardiac death protocols: a national survey. Transplantation. 2011;91(4):386–9.
Medicine, I.o., Non-heart-beating organ transplantation: medical and ethical issues in procurement, in non-heart-beating organ transplantation: medical and ethical issues in procurement. 1997, 1997 by the National Academy of Sciences: Washington, DC.
Sheth KN et al. Autoresuscitation after asystole in patients being considered for organ donation. Crit Care Med. 2012;40(1):158–61.
Bernat JL et al. Report of a National Conference on Donation after cardiac death. Am J Transplant. 2006;6(2):281–91.
Lewis J et al. Development of the University of Wisconsin donation After Cardiac Death Evaluation Tool. Prog Transplant. 2003;13(4):265–73.
DeVita MA et al. Donors after cardiac death: validation of identification criteria (DVIC) study for predictors of rapid death. Am J Transplant. 2008;8(2):432–41.
Yee AH et al. Factors influencing time to death after withdrawal of life support in neurocritical patients. Neurology. 2010;74(17):1380–5.
Rabinstein AA et al. Prediction of potential for organ donation after cardiac death in patients in neurocritical state: a prospective observational study. Lancet Neurol. 2012;11(5):414–9.
de Groot YJ et al. External validation of a prognostic model predicting time of death after withdrawal of life support in neurocritical patients. Crit Care Med. 2012;40(1):233–8.
He X et al. Nomogram for predicting time to death after withdrawal of life-sustaining treatment in patients with devastating neurological injury. Am J Transplant. 2015;15(8):2136–42. Of importance: First complete nomogram predicting time to death following withdrawal of life-sustaining therapies in neurocritically ill patients that included several neurologic variables.
He X et al. The development and validation of a nomogram for identification of potential donation after cardiac death donors. 2015. Am J Transplant.
Medicine, I.o., Non-heart-beating organ transplantation: practice and protocols, in non-heart-beating organ transplantation: practice and protocols. 2000, 2000 by the National Academy of Sciences: Washington, DC.
Reich DJ et al. ASTS recommended practice guidelines for controlled donation after cardiac death organ procurement and transplantation. Am J Transplant. 2009;9(9):2004–11.
Detry O et al. Categories of donation after cardiocirculatory death. Transplant Proc. 2012;44(5):1189–95.
Society, B.T. National standards for organ retrieval from deceased donors. 2013.
Abt P et al. Liver transplantation from controlled non-heart-beating donors: an increased incidence of biliary complications. Transplantation. 2003;75(10):1659–63.
Foley DP et al. Donation after cardiac death: the University of Wisconsin experience with liver transplantation. Ann Surg. 2005;242(5):724–31.
Lee KW et al. Factors affecting graft survival after liver transplantation from donation after cardiac death donors. Transplantation. 2006;82(12):1683–8.
Locke JE et al. Outcomes of kidneys from donors after cardiac death: implications for allocation and preservation. Am J Transplant. 2007;7(7):1797–807.
Phua J et al. Pro/con debate: in patients who are potential candidates for organ donation after cardiac death, starting medications and/or interventions for the sole purpose of making the organs more viable is an acceptable practice. Crit Care. 2007;11(2):211.
Mateos-Rodríguez AA. Kidney transplant function using organs from non-heart-beating donors maintained by mechanical chest compressions. Resuscitation. 2010;81(7):904–7.
Akoh JA. Kidney donation after cardiac death. World J Nephrol. 2012;1(3):79–91.
Wilkinson D, Savulescu J. Should we allow organ donation euthanasia? Alternatives for maximizing the number and quality of organs for transplantation. Bioethics. 2012;26(1):32–48.
Mollaret P, Goulon M. [The depassed coma (preliminary memoir)]. Rev Neurol (Paris). 1959;101:3–15.
Wertheimer P, Jouvet M, Descotes J. Diagnosis of death of the nervous system in comas with respiratory arrest treated by artificial respiration. Presse Med. 1959;67(3):87–8.
Wijdicks EF. Determining brain death in adults. Neurology. 1995;45(5):1003–11.
A.H. Committee. A definition of irreversible coma. Report of the Ad Hoc Committee of the Harvard Medical School to Examine the Definition of Brain Death. JAMA. 1968;205(6):337–40.
Wijdicks EF et al. Evidence-based guideline update: determining brain death in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2010;74(23):1911–8.
Transplant., N.B.a. Transplant activity in the UK. Activity report 2014/15. 2014.
OPTN/UNOS OPTN/UNOS OPO Committee Report. 2008.
de Groot YJ et al. Imminent brain death: point of departure for potential heart-beating organ donor recognition. Intensive Care Med. 2010;36(9):1488–94.
Wijdicks, E.F.M.E.F., Determining brain death. Continuum (Minneapolis, Minn.), 2015. 21(5 neurocritical care): pp. 1411-1424.
Hwang DY, Gilmore EJ, Greer DM. Assessment of brain death in the neurocritical care unit. Neurosurg Clin N Am. 2013;24(3):469–82.
Saposnik G et al. Problems associated with the apnea test in the diagnosis of brain death. Neurol India. 2004;52(3):342–5.
Denny JT et al. A new technique for avoiding barotrauma-induced complications in apnea testing for brain death. J Clin Neurosci. 2015;22(6):1021–4.
Paries M et al. Benefit of a single recruitment maneuver after an apnea test for the diagnosis of brain death. Crit Care. 2012;16(4):R116.
Solek-Pastuszka J et al. Evolution of apnoea test in brain death diagnostics. Anaesth Intensive Ther. 2015;47(4):363–7.
Wieczorek A, Gaszynski T. Boussignac CPAP system for brain death confirmation with apneic test in case of acute lung injury/adult respiratory distress syndrome—series of cases. Ther Clin Risk Manag. 2015;11:961–5.
Giani, M.M., Apnea test during brain death assessment in mechanically ventilated and ECMO patients. Intensive Care Med. 2015.
Anthony DC et al. The systemic response to brain injury and disease. Brain Behav Immun. 2012;26(4):534–40.
Watts RPRP. Inflammatory signalling associated with brain dead organ donation: from brain injury to brain stem death and posttransplant ischaemia reperfusion injury. J Transplant. 2013;2013(2):1–19.
Lee ST et al. Cholinergic anti-inflammatory pathway in intracerebral hemorrhage. Brain Res. 2010;1309:164–71.
Adrie C et al. Immune status and apoptosis activation during brain death. Shock. 2010;33(4):353–62.
Hoeger S et al. Modulation of brain dead induced inflammation by vagus nerve stimulation. Am J Transplant. 2010;10(3):477–89.
Skrabal CA et al. Organ-specific regulation of pro-inflammatory molecules in heart, lung, and kidney following brain death. J Surg Res. 2005;123(1):118–25.
Schrader H, Hall C, Zwetnow NN. Effects of prolonged supratentorial mass expansion on regional blood flow and cardiovascular parameters during the Cushing response. Acta Neurol Scand. 1985;72(3):283–94.
Busl KM, Bleck TP. Neurogenic pulmonary edema. Crit Care Med. 2015;43(8):1710–5.
Berman M et al. Is stress cardiomyopathy the underlying cause of ventricular dysfunction associated with brain death? J Heart Lung Transplant. 2010;29(9):957–65.
Salim AA. Complications of brain death: frequency and impact on organ retrieval. Am Surg. 2006;72(5):377.
Smith M. Physiologic changes during brain stem death—lessons for management of the organ donor. J Heart Lung Transplant. 2004;23(9 Suppl):S217–22.
Avlonitis VS et al. The hemodynamic mechanisms of lung injury and systemic inflammatory response following brain death in the transplant donor. Am J Transplant. 2005;5(4 Pt 1):684–93.
Chen EP et al. Hormonal and hemodynamic changes in a validated animal model of brain death. Crit Care Med. 1996;24(8):1352–9.
Klein AS et al. Organ donation and utilization in the United States, 1999–2008. Am J Transplant. 2010;10(4 Pt 2):973–86.
Westendorp WH, Leuvenink HG, Ploeg RJ. Brain death induced renal injury. Curr Opin Organ Transplant. 2011;16(2):151–6.
Ramjug S, Hussain N, Yonan N. Prolonged time between donor brain death and organ retrieval results in an increased risk of mortality in cardiac transplant recipients. Interact Cardiovasc Thorac Surg. 2011;12(6):938–42.
Dikdan GS, Mora-Esteves C, Koneru B. Review of randomized clinical trials of donor management and organ preservation in deceased donors: opportunities and issues. Transplantation. 2012;94(5):425–41.
Macdonald PS et al. A systematic review and meta-analysis of clinical trials of thyroid hormone administration to brain dead potential organ donors. Crit Care Med. 2012;40(5):1635–44.
Sharpe MDMD. Oral and intravenous thyroxine (T4) achieve comparable serum levels for hormonal resuscitation protocol in organ donors: a randomized double-blinded study. Can J Anesth. 2013;60(10):998–1002.
Pinsard M et al. Interest of low-dose hydrocortisone therapy during brain-dead organ donor resuscitation: the CORTICOME study. Crit Care. 2014;18(4):R158.
Kotloff RM et al. Management of the potential organ donor in the ICU: Society of Critical Care Medicine/American College of Chest Physicians/Association of Organ Procurement Organizations Consensus Statement. Crit Care Med. 2015;43(6):1291–325. First guidelines for the critical care management of the potential organ donor.
Mi Z et al. The optimal hormonal replacement modality selection for multiple organ procurement from brain-dead organ donors. Clin Epidemiol. 2015;7:17–27.
Hadian M et al. Cross-comparison of cardiac output trending accuracy of LiDCO, PiCCO, FloTrac and pulmonary artery catheters. Crit Care. 2010;14(6):R212.
Al-Khafaji A et al. Protocolized fluid therapy in brain-dead donors: the multicenter randomized MOnIToR trial. Intensive Care Med. 2015;41(3):418–26.
Wood KE et al. Care of the potential organ donor. N Engl J Med. 2004;351(26):2730–9.
Schnuelle P et al. Effects of donor pretreatment with dopamine on graft function after kidney transplantation: a randomized controlled trial. JAMA. 2009;302(10):1067–75.
Wang Z et al. Stimulation of dopamine D3 receptor attenuates renal ischemia-reperfusion injury via increased linkage with Galpha12. Transplantation. 2015;99(11):2274–84.
Mascia L et al. Effect of a lung protective strategy for organ donors on eligibility and availability of lungs for transplantation: a randomized controlled trial. JAMA. 2010;304(23):2620–7.
Minambres E et al. Lung donor treatment protocol in brain dead-donors: a multicenter study. J Heart Lung Transplant. 2015;34(6):773–80.
Minambres E et al. Impact of restrictive fluid balance focused to increase lung procurement on renal function after kidney transplantation. Nephrol Dial Transplant. 2010;25(7):2352–6.
Miñambres EE. Aggressive lung donor management increases graft procurement without increasing renal graft loss after transplantation. Clin Transpl. 2013;27(1):52–9.
Prodhan P et al. Inhaled nitric oxide in neurogenic cardiopulmonary dysfunction: implications for organ donation. Transplant Proc. 2004;36(9):2570–2.
Park ES et al. Inhaled nitric oxide for the brain dead donor with neurogenic pulmonary edema during anesthesia for organ donation: a case report. Korean J Anesthesiol. 2014;67(2):133–8.
Ware LBL. A randomized trial of the effects of nebulized albuterol on pulmonary edema in brain-dead organ donors. Am J Transplant. 2014;14(3):621.
Andres A et al. Lower rate of family refusal for organ donation in non-heart-beating versus brain-dead donors. Transplant Proc. 2009;41(6):2304–5.
Volk ML et al. Attitudes of the American public toward organ donation after uncontrolled (sudden) cardiac death. Am J Transplant. 2010;10(3):675–80.
Niemann CU et al. Therapeutic hypothermia in deceased organ donors and kidney-graft function. N Engl J Med. 2015;373(5):405–14.
Evrard P. Belgian modified classification of Maastricht for donors after circulatory death. Transplant Proc. 2014;46(9):3138–42.
Trasplantes, O.N.d. Donation after circulatory death in Spain: current situation and recommendations. National Consensus Document. 2012.
Dujardin KS et al. Myocardial dysfunction associated with brain death: clinical, echocardiographic, and pathologic features. J Heart Lung Transplant. 2001;20(3):350–7.
Gramm HJ et al. Acute endocrine failure after brain death? Transplantation. 1992;54(5):851–7.
Wind JJ. Preservation of kidneys from controlled donors after cardiac death. Br J Surg. 2011;98(9):1260–6.
Reznik ON et al. Uncontrolled donors with controlled reperfusion after sixty minutes of asystole: a novel reliable resource for kidney transplantation. PLoS One. 2013;8(5), e64209.
Barlow AD, Hosgood SA, Nicholson ML. Current state of pancreas preservation and implications for DCD pancreas transplantation. Transplantation. 2013;95(12):1419–24.
Morrissey PE, Monaco AP. Donation after circulatory death: current practices, ongoing challenges, and potential improvements. Transplantation. 2014;97(3):258–64.
Bejaoui M et al. Emerging concepts in liver graft preservation. World J Gastroenterol. 2015;21(2):396–407.
Dhital KK et al. Adult heart transplantation with distant procurement and ex-vivo preservation of donor hearts after circulatory death: a case series. Lancet. 2015;385(9987):2585–91.
Chaumont M et al. Delayed graft function in kidney transplants: time evolution, role of acute rejection, risk factors, and impact on patient and graft outcome. J Transplant. 2015;2015:163757.
Acknowledgments
Dr. Carolina B. Maciel receives research grant funding from Swebilius Foundation.
Dr. David M. Greer serves as Editor-in-Chief of Seminars in Neurology and has received compensation for medico-legal consultation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Carolina B. Maciel and David M. Greer declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Funding
None
Additional information
This article is part of the Topical Collection on Critical Care
Rights and permissions
About this article
Cite this article
Maciel, C.B., Greer, D.M. ICU Management of the Potential Organ Donor: State of the Art. Curr Neurol Neurosci Rep 16, 86 (2016). https://doi.org/10.1007/s11910-016-0682-1
Published:
DOI: https://doi.org/10.1007/s11910-016-0682-1