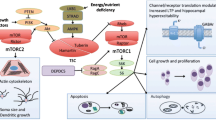
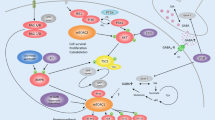
Abstract
New epilepsy treatments are needed that not only inhibit seizures symptomatically (antiseizure) but also prevent the development of epilepsy (antiepileptogenic). The mammalian target of rapamycin (mTOR) pathway may mediate mechanisms of epileptogenesis and serve as a rational therapeutic target. mTOR inhibitors have antiepileptogenic and antiseizure effects in animal models of the genetic disease, tuberous sclerosis complex. The mTOR pathway is also implicated in epileptogenesis in animal models of acquired epilepsy and infantile spasms, although the effects of mTOR inhibitors are variable depending on the specific conditions and model. Furthermore, beneficial effects on seizures are lost when treatment is withdrawn, suggesting that mTOR inhibitors are “epileptostatic” in only stalling epilepsy progression during treatment. Clinical studies of rapamycin in human epilepsy are limited, but suggest that mTOR inhibitors at least have antiseizure effects in tuberous sclerosis patients. Further studies are needed to assess the full potential of mTOR inhibitors for epilepsy treatment.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Crino PB, Nathanson KL, Henske EP. The tuberous sclerosis complex. N Engl J Med. 2006;355:1345–56.
McDaniel SS, Wong M. Therapeutic role of mammalian target of rapamycin (mTOR) inhibition in preventing epileptogenesis. Neurosci Lett. 2011;497:231–9.
Wong M. Mammalian target of rapamycin (mTOR) inhibition as a potential antiepileptogenic therapy: From tuberous sclerosis to common acquired epilepsies. Epilepsia. 2010;51:27–36.
Cho CH. Frontier of epilepsy research—mTOR signaling pathway. Exp Mol Med. 2011;43:231–74.
Weber JD, Gutmann DH. Deconvoluting mTOR biology. Cell Cycle. 2012;11:236–48.
Freeman JM, Kossoff EH. Ketosis and the ketogenic diet 2010: advances in treating epilepsy and other disorders. Adv Pediatr. 2010;57:315–29.
McDaniel SS, Rensing NR, Thio LL, Yamada KA, Wong M. The ketogenic diet inhibits the mammalian target of rapamycin (mTOR) pathway. Epilepsia. 2011;52:e7–e11.
Orlova KA, Parker WE, Heuer GG, Tsai V, Yoon J, Baybis M, Fenning RS, Strauss K, Crino PB. STRADalpha deficiency results in aberrant mTORC1 signaling during corticogenesis in humans and mice. J Clin Invest. 2010;120:1591–602.
Crino PB. Molecular pathogenesis of tuber formation in tuberous sclerosis complex. J Child Neurol. 2004;19:716–25.
• Krueger DA, Care MM, Holland K, Agricola K, et al. Everolimus for subependymal giant-cell astrocytomas in Tuberous Sclerosis. N Engl J Med. 2010;363:1801–11. This clinical trial showed that an mTOR inhibitor reduced the growth of subependymal giant cell astrocytomas in TSC patients, leading to FDA approval for this indication, and also provided some initial clinical data suggesting an effect of everolimus on seizures as a secondary measure.
Baybis M, Yu J, Lee A, Golden JA, Weiner H, McKhann G, Aronica E, Crino PB. mTOR cascade activation distinguishes tubers from focal cortical dysplasia. Ann Neurol. 2004;56:478–87.
Schick V, Majores M, Engels G, Hartmann W, Elger CE, Schramm J, Schoch S, Becker AJ. Differential Pi3K-pathway activation in cortical tubers and focal cortical dysplasias with balloon cells. Brain Pathol. 2007;17:165–73.
Becker AJ, Urbach H, Scheffler BJ, Baden T, Normann S, Lahl R, Pennek HW, Tuxhorn I, Elger CE, Schramm J, Wiestler OD, Blumcke I. Focal cortical dysplasia of Taylor’s balloon cell type: mutational analysis of the TSC1 gene indicates a pathogenic relationship to Tuberous Sclerosis. Ann Neurol. 2002;52:29–37.
Schonberger A, Niehusmann P, Urbach H, Majores M, Grote A, Holthausen H, Blumcke I, Deckert M, Becker AJ. Increased frequency of distinct TSC2 alleleic variants in focal cortical dysplasias with balloon cells and mineralization. Neuropathology. 2009;29:559–65.
Gumbinger C, Rohsbach CB, Schulze-Bonhage A, Korinthenberg R, Zentner J, Haffner M, Fauser S. Focal cortical dysplasia: a genotype-phenotype analysis of polymorphisms and mutations in the TSC genes. Epilepsia. 2009;50:1396–408.
Grajkowski W, Kotulska K, Matyja E, Larysz-Brysz M, Mandera M, Roszkowski M, Domanska-Pakilela D, Lewik-Kowalik J, Jozwiak S. Expression of tuberin and hamartin in tuberous sclerosis complex-associated and sporadic cortical dysplasia of Taylor’s balloon cell type. Folia Neuropathol. 2008;46:43–8.
Lugnier C, Majores M, Fassunke J, Pernhorst K, Niehusmann P, Simon M, Nellist M, Schoch S, Becker A. Hamartin variants that are frequent in focal dysplasias and cortical tubers have reduced tuberin binding and aberrant subcellular distribution in vitro. J Neuropathol Exp Neurol. 2009;68:1136–46.
Ljungberg MC, Bhattacharjee MB, Lu Y, Armstrong DL, Yoshor D, Swann JW, Sheldon M, D’Arcangelo G. Activation of mammalian target of rapamycin in cytomegalic neurons of human cortical dysplasia. Ann Neurol. 2006;60:420–9.
Miyata H, Chiang ACY, Vinters HV. Insulin signaling pathways in cortical dysplasia and TSC-tubers: tissue microarray analysis. Ann Neurol. 2004;56:510–9.
Uhlmann EJ, Wong M, Baldwin RL, Bajenaru ML, Onda H, Kwiatkowski DJ, Yamada KA, Gutmann DH. Astrocyte-specific TSC1 conditional knockout mice exhibit abnormal neuronal organization and seizures. Ann Neurol. 2002;52:285–96.
Meikle L, Talos DM, Onda H, Pollizzi K, Rotenberg A, Sahin M, Jensen FE, Kwiatkowski DJ. A mouse model of tuberous sclerosis: neuronal loss of Tsc1 causes dysplastic and ectopic neurons, reduced myelination, seizure activity, and limited survival. J Neurosci. 2007;27:5546–58.
• Goto J, Talos DM, Klein P, Qin W, Chekaluk YI, Anderl S, Malinowska IA, Di Nardo A, Bronson RT, Chan JA, Vinters HV, Kernie SG, Jensen FE, Sahin M, Kwiatkowski DJ. Regulable neural progenitor-specific TSC1 loss yields giant cells with organellar dysfunction in a model of tuberous sclerosis complex. PNAS. 2011;108:1070–9. This study described a novel mouse model of TSC and, consistent with previous studies, demonstrated that early postnatal treatment could prevent epilepsy and associated pathological abnormalities in these mice.
Carson RP, Van Nielen DL, Winzenburger PA, Ess KC. Neuronal and glia abnormalities in TSC1—deficient forebrain and partial rescue by rapamycin. Neurobiol Dis. 2012;45:369–80.
Fu C, Cawthon B, Clinkscales W, Bruce A, Winzenburger P, Ess KC. GABAergic interneuron development and function is modulated by the Tsc1 gene. Cereb Cortex 2011 Oct 20 Epub ahead of print
Way SW, McKenna 3rd J, Mietzsch U, Reith RM, Wu HC, Gambello MJ. Loss of Tsc2 in radial glia models the brain pathology of tuberous sclerosis complex in the mouse. Hum Mol Genet. 2009;18:1252–65.
Ehninger D, Han S, Shilyansky C, Zhou Y, Li W, Kwiatkowski DJ, Ramesh V, Silva AJ. Reversal of learning deficits in a TSC2 +/− mouse model of tuberous sclerosis. Nat Med. 2008;14:843–8.
Zeng L, Rensing NR, Zhang B, Gutmann DH, Gambello MJ, Wong M. TSC2 gene inactivation causes a more severe epilepsy phenotype than TSC1 inactivation in a mouse model of Tuberous Sclerosis Complex. Hum Mol Genet. 2011;20:445–54.
Zeng L, Xu L, Gutmann DH, Wong M. Rapamycin prevents epilepsy in a mouse model of Tuberous Sclerosis Complex. Ann Neurol. 2008;63:444–53.
Meikle L, Pollizzi K, Egnor A, Kramvis I, Lane H, Sahin M, Kwiatkowski. Response of a neuronal model of Tuberous Sclerosis to mammalian target of Rapamycin (mTOR) inhibitors: effects on mTORC1 and Akt signaling lead to improved survival and function. J Neurosci. 2008;28:5422–32.
Marsh DJ, Kum JB, Lunetta KL, Bennett MJ, et al. PTEN mutation spectrum and genotype - phenotype correlations in Bannayan–Riley–Ruvalcaba syndrome suggest a single entity with Cowden syndrome. Hum Mol Genet. 1999;8:1461–72.
Backman SA, Stambolic V, Suzuki A, Haight J, Elia A, Pretorius J, Tsao MS, Shannon P, Bolon B, Ivy GO, Mak TW. Deletion of Pten in mouse brain causes seizures, ataxia, and defects in soma size resembling Lhermitte-Duclos disease. Nat Genet. 2001;29:396–403.
Kwon CH, Zhu X, Zhang J, Knoop LL, Tharp R, Smeyne RJ, Eberhart CG, Burger PC, Baker SJ. Pten regulates neuronal soma size: a mouse model of Lhermitte-Duclos disease. Nat Genet. 2001;29:404–11.
Kwon C, Zhu X, Zhang J, Baker SJ. mTor is required for hypertrophy of Pten-deficient neuronal soma in vivo. Proc Natl Acad Sci USA. 2003;100:12923–8.
Ljungberg MC, Sunnen CN, Lugo JN, Anderson AE, D’Arcangelo G. Rapamycin suppresses seizures and neuronal hypertrophy in a mouse model of cortical dysplasia. Dis Model Mech. 2009;2:389–98.
• Sunnen CN, Brewster AL, Lugo JN, Vanegas F, Turclos E, Mukhi S, Parghi D, D’Arcangelo G, Anderson AE. Inhibition of the mammalian target of rapamycin blocks epilepsy progression in NS-Pten conditional knockout mice. Epilepsia. 2011;52:2065–75. This study demonstrated that intermittent (2 weeks on, 4 weeks off) treatment with rapamycin prevented epilepsy progression and reduced side effects on growth in a genetic model of epilepsy.
Zhou J, Blundell J, Ogawa S, Kwon C, Zhang W, Sinton C, Powell CM, Parada LF. Pharmacological inhibition of mTORC1 suppresses anatomical, cellular, and behavioral abnormalities in neural-specific Pten knock-out mice. J Neurosci. 2009;29:1773–83.
• Zeng LH, Rensing NR, Wong M. The mammalian target of rapamycin signaling pathway mediates epileptogenesis in a model of temporal lobe epilepsy. J Neurosci. 2009;29:6964–72. This study provided evidence that mTOR inhibitors may inhibit epilepsy development in an animal model of acquired epilepsy following status epilepticus.
Buckmaster PS, Ingram EA, Wen X. Inhibition of the mammalian target of rapamycin signaling pathway suppresses dentate granule cell axon sprouting in a rodent model of temporal lobe epilepsy. J Neurosci. 2009;29:8259–69.
Huang X, Zhang H, Yang J, Wu J, McMahon J, Lin Y, Cao Z, Gruenthal M, Huang Y. Pharmacological inhibition of the mammalian target of rapamycin pathway suppresses acquired epilepsy. Neurobiol Dis. 2010;40:193–9.
• Buckmaster PS, Lew FH. Rapamycin suppresses mossy fiber sprouting but not seizure frequency in a mouse model of temporal lobe epilepsy. J Neurosci. 2011;31:2337–47. This study demonstrated that rapamycin inhibits mossy fiber sprouting, but did not prevent epilepsy in a model of acquired temporal lobe epilepsy.
Sliwa A, Plucinska G, Bednarczyk J, Lukasiuk K. Post-treatment with rapamycin does not prevent epileptogenesis in the amygdala stimulation model of temporal lobe epilepsy. Neurosci Lett. 2012; Epub ahead of print
Wong M. Rapamycin for treatment of epilepsy: antiseizure, antiepileptogenic, both, or neither? Epilepsy Curr. 2011;11:66–8.
Temkin NR. Antiepileptogenesis and seizure prevention trials with antiepileptic drugs: meta-analysis of controlled trials. Epilepsia. 2001;42:515–24.
Chen S, Atkins CM, Liu CL, Alonso OF, Dietrich WD, Hu BR. Alterations in mammalian target of rapamycin signaling pathways after traumatic brain injury. J Cereb Blood Flow Metab. 2007;27:939–49.
Erlich S, Alexandrovich A, Shohami E, Pinkas-Kramarski R. Rapamycin is a neuroprotective treatment for traumatic brain injury. Neurobiol Dis. 2007;26:86–93.
Guo D, Zeng LH, Brody DL, Wong M. mTOR inhibition has potential antiepileptogenic effects in a controlled cortical impact model of traumatic brain injury. Epilepsy Curr. 2011;11(Suppl 1): Abstract #1.014
Park J, Zhang J, Qiu J, Zhu X, Degterev A, Lo EH, Whalen MJ. Combination therapy targeting Akt and mammalian target of rapamycin improves functional outcome after controlled cortical impact. J Cereb Blood Flow Metab. 2012;32:330–40.
Berdichevsky Y, Saponjian Y, Mail M, Staley KJ. Organotypic culture model of post-traumatic epileptogenesis used as a medium-throughput screen of antiepileptic drugs. Epilepsy Curr. 2012;12(Suppl 1): Abstract #3.026
• Raffo E, Coppola A, Ono T, Briggs SW, Galanopoulo AS. A pulse rapamycin therapy for infantile spasms and associated cognitive decline. Neurobiol Dis. 2011;43:322–8. This study found that rapamycin can suppress spasms and reduce cognitive deficits in an animal model of infantile spasms.
Chachua T, Yum MS, Veliskova J, Velisek L. Validation of the rat model of cryptogenic infantile spasms. Epilepsia. 2011;52:1666–77.
Talos DM, Sun H, Jackson M, Joseph A, Fitzgerald E, Jensen F. Rapamycin attenuates the increases in seizure susceptibility and neuronal excitability following neonatal seizures in rat. Epilepsy Curr. 2011;11(Suppl 1):Abstract A.08
Sankar R, Auvin S, Kwon YS, Pineda E, Shin D, Mazarati A. Evaluation of development-specific targets for antiepileptogenic therapy using rapid kindling. Epilepsia. 2010;51(Suppl3):39–42.
Muncy J, Butler IJ, Koenig M. Rapaymycin reduces seizure frequency in Tuberous Sclerosis Complex. J Child Neurol. 2009;24:477.
Wilfong A, Krueger DA, Holland-Bouley K, Anderson AE, Agricola K, Schultz RJ, Tudor C, Mayws M, Lopez CM, Franz DN. Everolimus improves seizure control in tuberous sclerosis complex. American Epilepsy Society Meeting Abstracts 2011; Late-breaking Abstract #3.329
Daoud D, Scheld HH, Speckmann EJ, Gorji A. Rapamycin: brain excitability studied in vitro. Epilepsia. 2007;48:834–6.
Ruegg S, Baybis M, Juul H, Dichter M, Crino PB. Effects of rapamycin on gene expression, morphology, and electrophysiological properties of rat hippocampal neurons. Epilepsy Res. 2007;77:85–92.
Galanopoulou A, Crino P, Wong M, Buckmaster P, Raffo E. Rapamycin: from tuberous sclerosis and beyond. Epilepsia. 2009;50 Suppl 11:309.
Anderl S, Freeland M, Kwiatkowski DJ, Goto J. Therapeutic value of prenatal rapamycin treatment in a mouse brain model of tuberous sclerosis complex. Hum Mol Genet. 2011;20:4597–604.
Guardia O, del Rial MC, Casadei D. Pregnancy under sirolimus-based immunosuppression. Transplantation. 2006;81:636.
Sifontis NM, Coscia LA, Constantinescu S, Lavelanet AF, Moritz MJ, Armenti VT. Pregnancy outcomes in solid organ transplant recipients with exposure to mycophenolate mofetil or sirolimus. Transplantation. 2006;82:1698–702.
Chu SH, Liu KL, Chiang YJ, Wang HH, Lai PC. Sirolimus used during pregnancy in a living related renal transplant recipient: a case report. Transplant Proc. 2008;40:2446–8.
Jozwiak S, Kotulsaka K, Domanska-Pakilea D, Lojszczyk B, Syczewska M, Chmielewski D, Dunin-Wasocwicz D, Kmiec T, Szymkiewicz-Dangel J, Kornacka M, Kawalec W, Kuczynski D, Borkowska J, Tomaszek K, Jurkiewicz E, Respondek-Liberska M. Antiepileptic treatment before the onset of seizures reduces epilepsy severity and risk of mental retardation in infants with tuberous sclerosis complex. Eur J Paediatr Neurol. 2011;15:424–31.
Acknowledgments
M. Wong receives funding from the National Institutes of Health (R01 NS056872) and the Washington University/Pfizer Biomedical Research Collaboration.
Disclosure
Conflicts of interest: R.C.C. Ryther: has received travel/accommodations/meeting expenses unrelated to activities listed from the American Epilepsy Society; M. Wong: none.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ryther, R.C.C., Wong, M. Mammalian Target of Rapamycin (mTOR) Inhibition: Potential for Antiseizure, Antiepileptogenic, and Epileptostatic Therapy. Curr Neurol Neurosci Rep 12, 410–418 (2012). https://doi.org/10.1007/s11910-012-0276-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11910-012-0276-5