Abstract
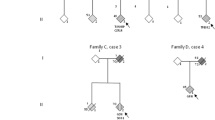
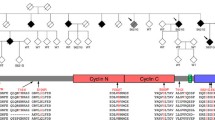
There is a clinical and pathological overlap between amyotrophic lateral sclerosis (ALS) and frontotemporal lobar degeneration (FTLD). A number of autosomal-dominant genes have been described that primarily cause ALS or FTLD such as progranulin (GRN), valosin-containing protein (VCP), and TAR DNA-Binding Protein (TARDBP), and for each of these conditions there are a small number of cases with both ALS and FTLD. Two major genes were described in 2011, which cause FTLD and/or ALS within extended kindreds. Ubiquilin2 (UBQLN2) is responsible for X-linked FTLD/ALS. A hexanucleotide repeat expansion in C9ORF72 causes chromosome 9p linked FTLD/ALS and is the most common cause of familial ALS accounting for about 40 % of familial cases. Both UBQLN2 and C9ORF72 mutations lead to TDP-43 positive neuropathology, and C9ORF72-positive cases have p62/ubiquitin-positive pathology, which is not stained by TDP-43 antibodies. Ubiquilin2 is one of a family of proteins thought to be important in targeting abnormal proteins for degradation via lysosomal and proteasomal routes. The pathogenic mechanism of the C9ORF72 expansion is unknown but may involve partial haploinsufficiency of C9ORF72 and/or the formations of toxic RNA inclusions. The identification of mutations in these genes represents an important step forward in our understanding of the clinical, pathological, and genetic spectrum of ALS/FTLD diseases.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as:•• Of major importance
Mitsuyama Y, Takamiya S. Presenile dementia with motor neuron disease in Japan. A new entity? Arch Neurol. 1979;36(9):592–3.
Wikström J, Paetau A, Palo J, Sulkava R, Haltia M. Classic amyotrophic lateral sclerosis with dementia. Arch Neurol. 1982;39(11):681–3.
Neary D, Snowden JS, Mann DM, Northen B, Goulding PJ, Macdermott N. Frontal lobe dementia and motor neuron disease. J Neurol Neurosurg Psychiatry. 1990;53(1):23–32.
Leigh PN, Whitwell H, Garofalo O, Buller J, Swash M, Martin JE, et al. Ubiquitin-immunoreactive intraneuronal inclusions in amyotrophic lateral sclerosis. Morphology, distribution, and specificity. Brain: J Neurol. 1991;114(Pt 2):775–88.
Okamoto K, Hirai S, Yamazaki T, Sun XY, Nakazato Y. New ubiquitin-positive intraneuronal inclusions in the extra-motor cortices in patients with amyotrophic lateral sclerosis. Neurosci Lett. 1991;129(2):233–6.
Jackson M, Lennox G, Lowe J. Motor neurone disease-inclusion dementia. Neurodegeneration: J Neurodegener Disord, Neuroprotection, Neuroregeneration. 1996;5(4):339–50.
Gunnarsson LG, Dahlbom K, Strandman E. Motor neuron disease and dementia reported among 13 members of a single family. Acta Neurol Scand. 1991;84(5):429–33.
Rossor MN, Revesz T, Lantos PL, Warrington EK. Semantic dementia with ubiquitin-positive tau-negative inclusion bodies. Brain: J Neurol. 2000;123(Pt 2):267–76.
Massman PJ, Sims J, Cooke N, Haverkamp LJ, Appel V, Appel SH. Prevalence and correlates of neuropsychological deficits in amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry. 1996;61(5):450–5.
Phukan J, Pender NP, Hardiman O. Cognitive impairment in amyotrophic lateral sclerosis. Lancet Neurol. 2007;6(11):994–1003.
Ringholz GM, Appel SH, Bradshaw M, Cooke Na, Mosnik DM, Schulz PE. Prevalence and patterns of cognitive impairment in sporadic ALS. Neurology. 2005;65(4):586–90.
Rademakers R, Cruts M, Dermaut B, Sleegers K, Rosso SM, Van den Broeck M, et al. Tau negative frontal lobe dementia at 17q21: significant finemapping of the candidate region to a 4.8 cM interval. Mol Psychiatr. 2002;7(10):1064–74.
Rademakers R, Rovelet-Lecrux A. Recent insights into the molecular genetics of dementia. Trends Neurosci. 2009;32(8):451–61.
Gass J, Cannon A, Mackenzie IR, Boeve B, Baker M, Adamson J, et al. Mutations in progranulin are a major cause of ubiquitin-positive frontotemporal lobar degeneration. Hum Mol Genet. 2006;15(20):2988–3001.
Chen-Plotkin AS, Martinez-Lage M, Sleiman PMa, Hu W, Greene R, Wood EM, et al. Genetic and clinical features of progranulin-associated frontotemporal lobar degeneration. Arch Neurol. 2011;68(4):488–97.
Neumann M, Sampathu DM, Kwong LK, Truax AC, Micsenyi MC, Chou TT, et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science (New York, NY). 2006;314(5796):130–3.
Polymenidou M, Lagier-Tourenne C, Hutt KR, Huelga SC, Moran J, Liang TY, et al. Long pre-mRNA depletion and RNA missplicing contribute to neuronal vulnerability from loss of TDP-43. Nat Neurosci. 2011;14(4):459–68.
Cairns NJ, Bigio EH, Mackenzie IRA, Neumann M, Lee VM-Y, Hatanpaa KJ, et al. Neuropathologic diagnostic and nosologic criteria for frontotemporal lobar degeneration: consensus of the consortium for frontotemporal lobar degeneration. Acta Neuropathol. 2007;114(1):5–22.
Weihl CC, Pestronk A, Kimonis VE. Valosin-containing protein disease: inclusion body myopathy with Paget’s disease of the bone and fronto-temporal dementia. Neuromuscul Disord: NMD. 2009;19(5):308–15.
Johnson JO, Mandrioli J, Benatar M, Abramzon Y, Van Deerlin VM, Trojanowski JQ, et al. Exome sequencing reveals VCP mutations as a cause of familial ALS. Neuron. 2010;68(5):857–64.
Watts GDJ, Wymer J, Kovach MJ, Mehta SG, Mumm S, Darvish D, et al. Inclusion body myopathy associated with Paget disease of bone and frontotemporal dementia is caused by mutant valosin-containing protein. Nat Genet. 2004;36(4):377–81.
Sreedharan J, Blair IP, Tripathi VB, Hu X, Vance C, Rogelj B, et al. TDP-43 mutations in familial and sporadic amyotrophic lateral sclerosis. Science (New York, NY). 2008;319(5870):1668–72.
Borroni B, Bonvicini C, Alberici A, Buratti E, Agosti C, Archetti S, et al. Mutation within TARDBP leads to frontotemporal dementia without motor neuron disease. Hum Mutat. 2009;30(11):E974–83.
Benajiba L, Le Ber I, Camuzat A, Lacoste M, Thomas-Anterion C, Couratier P, et al. TARDBP mutations in motoneuron disease with frontotemporal lobar degeneration. Ann Neurol. 2009;65(4):470–3.
Vance C, Rogelj B, Hortobágyi T, De Vos KJ, Nishimura AL, Sreedharan J, et al. Mutations in FUS, an RNA processing protein, cause familial amyotrophic lateral sclerosis type 6. Science (New York, NY). 2009;323(5918):1208–11.
Kwiatkowski TJ, Bosco DA, Leclerc AL, Tamrazian E, Vanderburg CR, Russ C, et al. Mutations in the FUS/TLS gene on chromosome 16 cause familial amyotrophic lateral sclerosis. Science (New York, NY). 2009;323(5918):1205–8.
Mackenzie IR, Rademakers R, Neumann M. TDP-43 and FUS in amyotrophic lateral sclerosis and frontotemporal dementia. Lancet Neurol. 2010;9(10):995–1007.
••Deng H-X, Chen W, Hong S-T, Boycott KM, Gorrie GH, Siddique N, et al. Mutations in UBQLN2 cause dominant X-linked juvenile and adult-onset ALS and ALS/dementia. Nature. 2011;477(7363):211–5. The first description of UBQLN2 as causing X-linked ALS, illustrating the effect of pathogenic mutations on proteasomal function, and the occurrence of ubiquilin2 pathology in sporadic ALS.
Millecamps S, Corcia P, Cazeneuve C, Boillée S, Seilhean D, Danel-Brunaud V, et al. Mutations in UBQLN2 are rare in French amyotrophic lateral sclerosis. Neurobiol Aging. 2011.
Zhang C, Saunders AJ. An emerging role for ubiquilin 1 in regulating protein quality control system and in disease pathogenesis. Discov Med. 2009;8(40):18–22.
Rothenberg C, Monteiro MJ. Ubiquilin at a crossroads in protein degradation pathways. Autophagy. 2010;6(7):979–80.
Rothenberg C, Srinivasan D, Mah L, Kaushik S, Peterhoff CM, Ugolino J, et al. Ubiquilin functions in autophagy and is degraded by chaperone-mediated autophagy. Hum Mol Genet. 2010;19(16):3219–32.
Morita M, Al-Chalabi A, Andersen PM, Hosler B, Sapp P, Englund E, et al. A locus on chromosome 9p confers susceptibility to ALS and frontotemporal dementia. Neurology. 2006;66(6):839–44.
Vance C, Al-Chalabi A, Ruddy D, Smith BN, Hu X, Sreedharan J, et al. Familial amyotrophic lateral sclerosis with frontotemporal dementia is linked to a locus on chromosome 9p13.2–21.3. Brain: J Neurol. 2006;129(Pt 4):868–76.
Pearson JP, Williams NM, Majounie E, Waite A, Stott J, Newsway V, et al. Familial frontotemporal dementia with amyotrophic lateral sclerosis and a shared haplotype on chromosome 9p. J Neurol. 2011;258(4):647–55.
Boxer AL, Mackenzie IR, Boeve BF, Baker M, Seeley WW, Crook R, et al. Clinical, neuroimaging and neuropathological features of a new chromosome 9p-linked FTD-ALS family. J Neurol Neurosurg Psychiatry. 2011;82(2):196–203.
Shatunov A, Mok K, Newhouse S, Weale ME, Smith B, Vance C, et al. Chromosome 9p21 in sporadic amyotrophic lateral sclerosis in the UK and seven other countries: a genome-wide association study. Lancet Neurol. 2010;9(10):986–94.
Laaksovirta H, Peuralinna T, Schymick JC, Scholz SW, Lai S-L, Myllykangas L, et al. Chromosome 9p21 in amyotrophic lateral sclerosis in Finland: a genome-wide association study. Lancet Neurol. 2010;9(10):978–85.
Mok K, Traynor BJ, Schymick J, Tienari PJ, Laaksovirta H, Peuralinna T, et al. Chromosome 9 ALS and FTD locus is probably derived from a single founder. Neurobiol Aging. 2012;33(1):209.e3–8.
•• Renton AE, Majounie E, Waite A, Simón-Sánchez J, Rollinson S, Gibbs JR, et al. A hexanucleotide repeat expansion in C9ORF72 is the cause of chromosome 9p21-linked ALS-FTD. Neuron. 2011;72(2):257–68. The first description of pathogenic mutations in C9orf72 in families with FTLD/ALS, in back-to-back publications with DeJesus-Hernandez et al. [41••]. Together with Mok et al. (39), illustrates that the hexanucleotide expansion occurs on a common haplotype, and shows that there is an exceptionally high rate of mutations in Finnish ALS patients.
•• DeJesus-Hernandez M, Mackenzie IR, Boeve BF, Boxer AL, Baker M, Rutherford NJ, et al. Expanded GGGGCC hexanucleotide repeat in noncoding region of C9ORF72 causes chromosome 9p-linked FTD and ALS. Neuron. 2011;72(2):1–12. The first description of pathogenic mutations in C9orf72 in families with FTLD/ALS, in back-to-back publications with Renton et al. [40••]. Describes haploinsufficiency of some isoforms of C9ORF72 and the occurrence of nuclear RNA inclusions.
Murray ME, DeJesus-Hernandez M, Rutherford NJ, Baker M, Duara R, Graff-Radford NR, et al. Clinical and neuropathologic heterogeneity of c9FTD/ALS associated with hexanucleotide repeat expansion in C9ORF72. Acta Neuropathol. 2011;122(6):673–90.
Snowden JS, Rollinson S, Thompson JC, Harris JM, Stopford CL, Richardson AMT, et al. Distinct clinical and pathological characteristics of frontotemporal dementia associated with C9ORF72 mutations. Brain: J Neurol. 2012;135(Pt 3):693–708.
Majounie E, Abramzon Y, Renton AE, Perry R, Bassett SS, Pletnikova O, et al. Repeat expansion in C9ORF72 in Alzheimer’s disease. N Engl J Med. 2012;366:283–4.
Gijselinck I, Van Langenhove T, van der Zee J, Sleegers K, Philtjens S, Kleinberger G, et al. A C9orf72 promoter repeat expansion in a Flanders-Belgian cohort with disorders of the frontotemporal lobar degeneration-amyotrophic lateral sclerosis spectrum: a gene identification study. Lancet Neurol. 2012;11(1):54–65.
Simón-Sánchez J, Dopper EGP, Cohn-Hokke PE, Hukema RK, Nicolaou N, Seelaar H, et al. The clinical and pathological phenotype of C9ORF72 hexanucleotide repeat expansions. Brain: J Neurol. 2012;135(Pt 3):723–35.
Todd PK, Paulson HL. RNA-mediated neurodegeneration in repeat expansion disorders. Ann Neurol. 2010;67(3):291–300.
Disclosure
Conflicts of interest: H.R. Morris: has board membership with GlaxoSmithKline, Orion, Boehringer, Abbott, Solvay, and TEVA; has received grant support from Medical Research Council, Ipsen Fund, Welsh Assembly Government, Parkinson’s UK; has received payment for lectures including service on speakers bureaus from UCB Pharma, Orion, Boehringer, GlaxoSmithKline, TEVA; has received travel/accommodations/meeting expenses unrelated to activities listed from UCB, Orion, GlaxoSmithKline, Boehringer, TEVA; A.J. Waite: has received grant support from Motor Neuron Disease Association (MNDA), and has received support for travel to meetings for the study or other purposes from MNDA and Guarantors of brain; N.M. Williams: has received grant support from MNDA; J.W. Neal: none; D.J. Blake: has received grant support from MNDA.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Morris, H.R., Waite, A.J., Williams, N.M. et al. Recent Advances in the Genetics of the ALS-FTLD Complex. Curr Neurol Neurosci Rep 12, 243–250 (2012). https://doi.org/10.1007/s11910-012-0268-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11910-012-0268-5