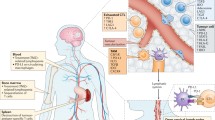
Abstract
Malignant glioma is a deadly disease for which there have been few therapeutic advances over the past century. Although previous treatments were largely unsuccessful, glioma may be an ideal target for immune-based therapy. Recently, translational research led to several clinical trials based on tumor immunotherapy to treat patients with malignant glioma. Here we review 17 recent glioma immunotherapy clinical trials, published over the past 3 years. Various approaches were used, including passive transfer of naked and radiolabeled antibodies, tumor antigen-specific peptide immunization, and the use of patient tumor cells with or without dendritic cells as vaccines. We compare and discuss the current state of the art of clinical immunotherapy treatment, as well as its limited successes, pitfalls, and future potential.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
United States Cancer Statistics: National Program of Cancer Registries. Available at http://apps.nccd.cdc.gov/uscs/.
Stupp R, Mason WP, van den Bent MJ, et al.: Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 2005, 352:987–996.
Walker MD, Green SB, Byar DP, et al.: Randomized comparisons of radiotherapy and nitrosoureas for the treatment of malignant glioma after surgery. N Engl J Med 1980, 303:1323–1329.
Brem H, Piantadosi S, Burger PC, et al.: Placebo-controlled trial of safety and efficacy of intraoperative controlled delivery by biodegradable polymers of chemotherapy for recurrent gliomas. The Polymer-brain Tumor Treatment Group. Lancet 1995, 345:1008–1012.
Westphal M, Hilt DC, Bortey E, et al.: A phase 3 trial of local chemotherapy with biodegradable carmustine (BCNU) wafers (Gliadel wafers) in patients with primary malignant glioma. Neuro Oncol 2003, 5:79–88.
Stummer W, Pichlmeier U, Meinel T, et al.: Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncol 2006, 7:392–401.
Friedman HS, Prados MD, Wen PY, et al.: Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol 2009, 27:4733–4740.
Mitchell DA, Fecci PE, Sampson JH: Immunotherapy of malignant brain tumors. Immunol Rev 2008, 222:70–100.
Rosenberg SA: Progress in human tumour immunology and immunotherapy. Nature 2001, 411:380–384.
Rosenberg SA, Yang JC, Restifo NP: Cancer immunotherapy: moving beyond current vaccines. Nat Med 2004, 10:909–915.
Dudley ME, Wunderlich JR, Robbins PF, et al.: Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science 2002, 298:850–854.
• Johnson LA, Morgan RA, Dudley ME, et al.: Gene therapy with human and mouse T-cell receptors mediates cancer regression and targets normal tissues expressing cognate antigen. Blood 2009, 114:535–546. This study demonstrated that ex vivo genetically engineered T cells can eliminate tumors in patients with advanced metastatic disease.
Morgan RA, Dudley ME, Wunderlich JR, et al.: Cancer regression in patients after transfer of genetically engineered lymphocytes. Science 2006, 314:126–129.
Harris M: Monoclonal antibodies as therapeutic agents for cancer. Lancet Oncol 2004, 5:292–302.
Neyns B, Sadones J, Joosens E, et al.: Stratified phase II trial of cetuximab in patients with recurrent high-grade glioma. Ann Oncol 2009, 20:1596–1603.
Casaco A, Lopez G, Garcia I, et al.: Phase I single-dose study of intracavitary-administered Nimotuzumab labeled with 188 Re in adult recurrent high-grade glioma. Cancer Biol Ther 2008, 7:333–339.
• Zalutsky MR, Reardon DA, Akabani G, et al.: Clinical experience with alpha-particle emitting 211At: treatment of recurrent brain tumor patients with 211At-labeled chimeric antitenascin monoclonal antibody 81C6. J Nucl Med 2008, 49:30–38. This preliminary clinical trial suggested improved patient survival compared with historical controls.
Yung WK, Albright RE, Olson J, et al.: A phase II study of temozolomide vs. procarbazine in patients with glioblastoma multiforme at first relapse. Br J Cancer 2000, 83:588–593.
Vredenburgh JJ, Desjardins A, Herndon JE 2nd, et al.: Phase II trial of bevacizumab and irinotecan in recurrent malignant glioma. Clin Cancer Res 2007, 13:1253–1259.
Chiocca EA, Smith KM, McKinney B, et al.: A phase I trial of Ad.hIFN-beta gene therapy for glioma. Mol Ther 2008, 16:618–626.
Groves MD, Puduvalli VK, Gilbert MR, et al.: Two phase II trials of temozolomide with interferon-alpha2b (pegylated and non-pegylated) in patients with recurrent glioblastoma multiforme. Br J Cancer 2009, 101:615–620.
Olson JJ, McKenzie E, Skurski-Martin M, et al.: Phase I analysis of BCNU-impregnated biodegradable polymer wafers followed by systemic interferon alfa-2b in adults with recurrent glioblastoma multiforme. J Neurooncol 2008, 90:293–299.
Hau P, Jachimczak P, Schlingensiepen R, et al.: Inhibition of TGF-beta2 with AP 12009 in recurrent malignant gliomas: from preclinical to phase I/II studies. Oligonucleotides 2007, 17:201–212.
Butowski N, Chang SM, Junck L, et al.: A phase II clinical trial of poly-ICLC with radiation for adult patients with newly diagnosed supratentorial glioblastoma: a North American Brain Tumor Consortium (NABTC01-05). J Neurooncol 2009, 91:175–182.
Butowski N, Lamborn KR, Lee BL, et al.: A North American brain tumor consortium phase II study of poly-ICLC for adult patients with recurrent anaplastic gliomas. J Neurooncol 2009, 91:183–189.
De Vleeschouwer S, Fieuws S, Rutkowski S, et al.: Postoperative adjuvant dendritic cell-based immunotherapy in patients with relapsed glioblastoma multiforme. Clin Cancer Res 2008, 14:3098–3104.
Ishikawa E, Tsuboi K, Yamamoto T, et al.: Clinical trial of autologous formalin-fixed tumor vaccine for glioblastoma multiforme patients. Cancer Sci 2007, 98:1226–1233.
Okada H, Lieberman FS, Walter KA, et al.: Autologous glioma cell vaccine admixed with interleukin-4 gene transfected fibroblasts in the treatment of patients with malignant gliomas. J Transl Med 2007, 5:67.
Walker DG, Laherty R, Tomlinson FH, et al.: Results of a phase I dendritic cell vaccine trial for malignant astrocytoma: potential interaction with adjuvant chemotherapy. J Clin Neurosci 2008, 15:114–121.
Wheeler CJ, Black KL, Liu G, et al.: Vaccination elicits correlated immune and clinical responses in glioblastoma multiforme patients. Cancer Res 2008, 68:5955–5964.
Izumoto S, Tsuboi A, Oka Y, et al.: Phase II clinical trial of Wilms tumor 1 peptide vaccination for patients with recurrent glioblastoma multiforme. J Neurosurg 2008, 108:963–971.
• Prins RM, Cloughesy TF, Liau LM: Cytomegalovirus immunity after vaccination with autologous glioblastoma lysate. N Engl J Med 2008, 359:539–541. The authors present a clinical observation of CMV-reactive T cells induced in a patient by vaccination with glioma tumor.
• Sampson JH, Archer GE, Mitchell DA, et al.: An epidermal growth factor receptor variant III-targeted vaccine is safe and immunogenic in patients with glioblastoma multiforme. Mol Cancer Ther 2009, 8:2773–2779. This preliminary clinical trial suggested improved patient survival compared with historical controls.
Cobbs CS, Harkins L, Samanta M, et al.: Human cytomegalovirus infection and expression in human malignant glioma. Cancer Res 2002, 62:3347–3350.
Mitchell DA, Xie W, Schmittling R, et al.: Sensitive detection of human cytomegalovirus in tumors and peripheral blood of patients diagnosed with glioblastoma. Neuro Oncol 2008, 10:10–18.
Lamborn KR, Yung WK, Chang SM, et al.: Progression-free survival: an important end point in evaluating therapy for recurrent high-grade gliomas. Neuro Oncol 2008, 10:162–170.
Johnson LA, Heemskerk B, Powell DJ Jr, et al.: Gene transfer of tumor-reactive TCR confers both high avidity and tumor reactivity to nonreactive peripheral blood mononuclear cells and tumor-infiltrating lymphocytes. J Immunol 2006, 177:6548–6559.
Lyman MA, Nugent CT, Marquardt KL, et al.: The fate of low affinity tumor-specific CD8+ T cells in tumor-bearing mice. J Immunol 2005, 174:2563–2572.
Humphrey PA, Wong AJ, Vogelstein B, et al.: Anti-synthetic peptide antibody reacting at the fusion junction of deletion-mutant epidermal growth factor receptors in human glioblastoma. Proc Natl Acad Sci U S A 1990, 87:4207–4211.
Sugawa N, Ekstrand AJ, James CD, Collins VP: Identical splicing of aberrant epidermal growth factor receptor transcripts from amplified rearranged genes in human glioblastomas. Proc Natl Acad Sci U S A 1990, 87:8602–8606.
Wikstrand CJ, Hale LP, Batra SK, et al.: Monoclonal antibodies against EGFRvIII are tumor specific and react with breast and lung carcinomas and malignant gliomas. Cancer Res 1995, 55:3140–3148.
Eshhar Z, Waks T, Gross G, Schindler DG: Specific activation and targeting of cytotoxic lymphocytes through chimeric single chains consisting of antibody-binding domains and the gamma or zeta subunits of the immunoglobulin and T-cell receptors. Proc Natl Acad Sci U S A 1993, 90:720–724.
Pule MA, Savoldo B, Myers GD, et al.: Virus-specific T cells engineered to coexpress tumor-specific receptors: persistence and antitumor activity in individuals with neuroblastoma. Nat Med 2008, 14:1264–1270.
Savoldo B, Rooney CM, Di Stasi A, et al.: Epstein Barr virus specific cytotoxic T lymphocytes expressing the anti-CD30zeta artificial chimeric T-cell receptor for immunotherapy of Hodgkin disease. Blood 2007, 110:2620–2630.
Disclosure
Dr. Sampson has stock options with Celldex Therapeutics and also has received consulting and license fees from that company. No other potential conflicts of interest relevant to this article were reported.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Johnson, L.A., Sampson, J.H. Immunotherapy Approaches for Malignant Glioma From 2007 to 2009. Curr Neurol Neurosci Rep 10, 259–266 (2010). https://doi.org/10.1007/s11910-010-0111-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11910-010-0111-9