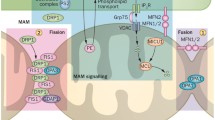
Abstract
Mitochondria are key organelles in eukaryotic cells that not only generate adenosine triphosphate but also perform such critical functions as hosting essential biosynthetic pathways, calcium buffering, and apoptotic signaling. In vivo, mitochondria form dynamic networks that undergo frequent morphologic changes through fission and fusion. In neurons, the imbalance of mitochondrial fission/fusion can influence neuronal physiology, such as synaptic transmission and plasticity, and affect neuronal survival. Core components of the mitochondrial fission/fusion machinery have been identified through genetic studies in model organisms. Mutations in some of these genes in humans have been linked to rare neurodegenerative diseases such as Charcot-Marie-Tooth subtype 2A and autosomal dominant optic atrophy. Recent studies also have implicated aberrant mitochondrial fission/fusion in the pathogenesis of more common neurodegenerative diseases such as Parkinson’s disease. These studies establish mitochondrial dynamics as a new paradigm for neurodegenerattve disease research. Compounds that modulate mitochondrial fission/fusion could have therapeutic value in disease intervention.
Similar content being viewed by others
References and Recommended Reading
Wallace DC: A mitochondrial paradigm of metabolic and degenerative diseases, aging, and cancer: a dawn for evolutionary medicine. Annu Rev Genet 2005, 39:359–407.
Okamoto K, Shaw JM: Mitochondrial morphology and dynamics in yeast and multicellular eukaryotes. Annu Rev Genet 2005, 39:503–536.
Mozdy AD, McCaffery JM, Shaw JM: Dnm1p GTPasemediated mitochondrial fission is a multi-step process requiring the novel integral membrane component Fis1p. J Cell Biol 2000, 151:367–380.
Tondera D, Czauderna F, Paulick K, et al.: The mitochondrial protein MTP18 contributes to mitochondrial fission in mammalian cells. J Cell Sci 2005, 118(Pt 14):3049–3059.
Koshiba T, Detmer SA, Kaiser JT, et al.: Structural basis of mitochondrial tethering by mitofusin complexes. Science 2004, 305:858–862.
Cipolat S, Martins de Brito O, Dal Zilio B, et al.: OPA1 requires mitofusin 1 to promote mitochondrial fusion. Proc Natl Acad Sci U S A 2004, 101:15927–15932.
Taguchi N, Ishihara N, Jofuku A, et al.: Mitotic phosphorylation of dynamin-related GTPase Drp1 participates in mitochondrial fission. J Biol Chem 2007, 282:11521–11529.
Cribbs JT, Strack S: Reversible phosphorylation of Drp1 by cyclic AMP-dependent protein kinase and calcineurin regulates mitochondrial fission and cell death. EMBO Rep 2007, 8:939–944.
Chang CR, Blackstone C: Cyclic AMP-dependent protein kinase phosphorylation of Drp1 regulates its GTPase activity and mitochondrial morphology. J Biol Chem 2007, 282:21583–21587.
Harder Z, Zunino R, McBride H: Sumo1 conjugates mitochondrial substrates and participates in mitochondrial fission. Curr Biol 2004, 14:340–345.
Karbowski M, Neutzner A, Youle RJ: The mitochondrial E3 ubiquitin ligase MARCH5 is required for Drp1 dependent mitochondrial division. J Cell Biol 2007, 178:71–84.
McQuibban GA, Saurya S, Freeman M: Mitochondrial membrane remodelling regulated by a conserved rhomboid protease. Nature 2003, 423:537–541.
Jeyaraju DV, Xu L, Letellier MC, et al.: Phosphorylation and cleavage of presenilin-associated rhomboid-like protein (PARL) promotes changes in mitochondrial morphology. Proc Natl Acad Sci U S A 2006, 103:18562–18567.
Benard G, Bellance N, James D, et al.: Mitochondrial bioenergetics and structural network organization. J Cell Sci 2007, 120(Pt 5):838–848.
Jendrach M, Pohl S, Voth M, et al.: Morpho-dynamic changes of mitochondria during ageing of human endothelial cells. Mech Ageing Dev 2005, 126:813–821.
Arakaki N, Nishihama T, Kohda A, et al.: Regulation of mitochondrial morphology and cell survival by mitogenin I and mitochondrial single-stranded DNA binding protein. Biochim Biophys Acta 2006, 1760:1364–1372.
Ichishita R, Tanaka K, Sugiura Y, et al.: An RNAi screen for mitochondrial proteins required to maintain the morphology of the organelle in Caenorhabditis elegans. J Biochem 2008, 143:449–454.
Altmann K, Westermann B: Role of essential genes in mitochondrial morphogenesis in Saccharomyces cerevisiae. Mol Biol Cell 2005, 16:5410–5417.
Frank S, Gaume B, Bergmann-Leitner ES, et al.: The role of dynamin-related protein 1, a mediator of mitochondrial fission, in apoptosis. Dev Cell 2001, 1:515–525.
Szabadkai G, Simoni AM, Chami M, et al.: Drp-1-dependent division of the mitochondrial network blocks intraorganellar Ca2+ waves and protects against Ca2+-mediated apoptosis. Mol Cell 2004, 16:59–68.
Huang P, Yu T, Yoon Y: Mitochondrial clustering induced by overexpression of the mitochondrial fusion protein Mfn2 causes mitochondrial dysfunction and cell death. Eur J Cell Biol 2007, 86:289–302.
Youle RJ, Karbowski M: Mitochondrial fission in apoptosis. Nat Rev Mol Cell Biol 2005, 6:657–663.
Kijima K, Numakura C, Izumino H, et al.: Mitochondrial GTPase mitofusin 2 mutation in Charcot-Marie-Tooth neuropathy type 2A. Hum Genet 2005, 116:23–27.
Alexander C, Votruba M, Pesch UE, et al.: OPA1, encoding a dynamin-related GTPase, is mutated in autosomal dominant optic atrophy linked to chromosome 3q28. Nat Genet 2000, 26:211–215.
Waterham HR, Koster J, van Roermund CW, et al.: A lethal defect of mitochondrial and peroxisomal fission. N Engl J Med 2007, 356:1736–1741.
Baxter RV, Ben Othmane K, Rochelle JM, et al.: Ganglioside-induced differentiation-associated protein-1 is mutant in Charcot-Marie-Tooth disease type 4A/8q21. Nat Genet 2002, 30:21–22.
Detmer SA, Chan DC: Complementation between mouse Mfn1 and Mfn2 protects mitochondrial fusion defects caused by CMT2A disease mutations. J Cell Biol 2007, 176:405–414.
Baloh RH, Schmidt RE, Pestronk A, et al.: Altered axonal mitochondrial transport in the pathogenesis of Charcot-Marie-Tooth disease from mitofusin 2 mutations. J Neurosci 2007, 27:422–430.
de Brito OM, Scorrano L: Mitofusin 2 tethers endoplasmic reticulum to mitochondria. Nature 2008, 456:605–610.
Bonifati V: Genetics of parkinsonism. Parkinsonism Relat Disord 2007, 13(Suppl 3):S233–S241.
Yang Y, Gehrke S, Imai Y, et al.: Mitochondrial pathology and muscle and dopaminergic neuron degeneration caused by inactivation of Drosophila Pink1 is rescued by Parkin. Proc Natl Acad Sci U S A 2006, 103:10793–10798.
Park J, Lee SB, Lee S, et al.: Mitochondrial dysfunction in Drosophila PINK1 mutants is complemented by parkin. Nature 2006, 441:1157–1161.
Clark IE, Dodson MW, Jiang C, et al.: Drosophila pink1 is required for mitochondrial function and interacts genetically with parkin. Nature 2006, 441:1162–1166.
Yang Y, Ouyang Y, Yang L, et al.: Pink1 regulates mitochondrial dynamics through interaction with the fission/fusion machinery. Proc Natl Acad Sci U S A 2008, 105:7070–7075.
Kim Y, Park J, Kim S, et al.: PINK1 controls mitochondrial localization of Parkin through direct phosphorylation. Biochem Biophys Res Commun 2008, 377:975–980.
Exner N, Treske B, Paquet D, et al.: Loss-of-function of human PINK1 results in mitochondrial pathology and can be rescued by parkin. J Neurosci 2007, 27:12413–12418.
Plun-Favreau H, Klupsch K, Moisoi N, et al.: The mitochondrial protease HtrA2 is regulated by Parkinson’s disease-associated kinase PINK1. Nat Cell Biol 2007, 9:1243–1252.
Whitworth AJ, Lee JR, Ho VM, et al.: Rhomboid-7 and HtrA2/Omi act in a common pathway with the Parkinson’s disease factors Pink1 and Parkin. Dis Model Mech 2008, 1:168–174.
Gomez-Lazaro M, Bonekamp NA, Galindo MF, et al.: 6-Hydroxydopamine (6-OHDA) induces Drp1-dependent mitochondrial fragmentation in SH-SY5Y cells. Free Radic Biol Med 2008, 44:1960–1969.
Dagda RK, Merrill RA, Cribbs JT, et al.: The spinocerebellar ataxia 12 gene product and protein phosphatase 2A regulatory subunit Bbeta2 antagonizes neuronal survival by promoting mitochondrial fission. J Biol Chem 2008, 283:36241–36248.
Wang X, Su B, Siedlak SL, et al.: Amyloid-beta overproduction causes abnormal mitochondrial dynamics via differential modulation of mitochondrial fission/fusion proteins. Proc Natl Acad Sci U S A 2008, 105:19318–19323.
McGuire JR, Rong J, Li SH, et al.: Interaction of Huntingtin-associated protein-1 with kinesin light chain: implications in intracellular trafficking in neurons. J Biol Chem 2006, 281:3552–3559.
Varadi A, Johnson-Cadwell LI, Cirulli V, et al.: Cytoplasmic dynein regulates the subcellular distribution of mitochondria by controlling the recruitment of the fission factor dynaminrelated protein-1. J Cell Sci 2004, 117(Pt 19):4389–4400.
Chee FC, Mudher A, Cuttle MF, et al.: Over-expression of tau results in defective synaptic transmission in Drosophila neuromuscular junctions. Neurobiol Dis 2005, 20:918–928.
Li Z, Okamoto K, Hayashi Y, et al.: The importance of dendritic mitochondria in the morphogenesis and plasticity of spines and synapses. Cell 2004, 119:873–887.
Stowers RS, Megeath LJ, Gorska-Andrzejak J, et al.: Axonal transport of mitochondria to synapses depends on milton, a novel Drosophila protein. Neuron 2002, 36:1063–1077.
Guo X, Macleod GT, Wellington A, et al.: The GTPase dMiro is required for axonal transport of mitochondria to Drosophila synapses. Neuron 2005, 47:379–393.
Almeida A, Almeida J, Bolanos JP, et al.: Different responses of astrocytes and neurons to nitric oxide: the role of glycolytically generated ATP in astrocyte protection. Proc Natl Acad Sci U S A 2001, 98:15294–15299.
Liang CL, Wang TT, Luby-Phelps K, et al.: Mitochondria mass is low in mouse substantia nigra dopamine neurons: implications for Parkinson’s disease. Exp Neurol 2007, 203:370–380.
Twig G, Elorza A, Molina AJ, et al.: Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. EMBO J 2008, 27:433–446.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lu, B. Mitochondrial dynamics and neurodegeneration. Curr Neurol Neurosci Rep 9, 212–219 (2009). https://doi.org/10.1007/s11910-009-0032-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11910-009-0032-7