Abstract
Community-acquired pneumonia (CAP) has a significant impact on public health in terms of short-term and long-term morbidity and mortality. Irrespective of microbiological etiology, the host’s inability to fully downregulate systemic inflammation is the dominant pathogenetic process contributing to acute and long-term morbidity and mortality in CAP. Glucocorticoids are the natural regulators of inflammation, and their production increases during infection. There is consistent evidence that downregulation of systemic inflammation with prolonged low-dose glucocorticoid treatment in patients with severe sepsis and acute respiratory distress syndrome improves cardiovascular and pulmonary organ physiology. A recent meta-analysis of pooled controlled small trials (n = 970) of patients admitted with CAP found improved short-term mortality in the subgroup with severe CAP and/or receiving >5 days of glucocorticoid treatment. We have expanded on this meta-analysis by including patients with CAP recruited in trials investigating prolonged low-dose glucocorticoid treatment in septic shock and/or early acute respiratory distress syndrome (n = 1,206). Our findings confirm a survival advantage for severe CAP (RR 0.66, 95% confidence interval 0.51–0.84; p = .001). A large randomized trial is in progress to confirm the aggregate findings of these small trials and to evaluate the long-term effect of this low-cost treatment.
Similar content being viewed by others
Short- and Long-Term Impact of Community-Acquired Pneumonia on Morbidity, Mortality, and Health-Care Cost
Community-acquired pneumonia (CAP) is a common infection with a wide spectrum of clinical severity ranging from a self-limiting illness to life-threatening septic shock, acute respiratory distress syndrome (ARDS), and multiple organ dysfunction. While mortality for pneumonia has decreased sharply following the introduction of antibiotics in the 1940s, since 1950, the overall acute (hospital) mortality has either remained stable or increased [1]. Acute mortality (≈4%–6% during initial hospitalization) makes pneumonia, with ≈55,000 deaths per year, the eighth most common cause of death in the U.S. [2]. Most hospital deaths, however, occur after eradication of bacteria from tracheal secretions and blood stream [3, 4], implying that adequate antibiotic treatment alone may be insufficient in achieving additional decline in morbidity and mortality. Survivors experience substantial and persistent new cognitive impairment and functional disability [5–12, 13••, 14–17]. This excess disability and mortality after the original hospitalization for pneumonia extends for years [15–21]. This new awareness calls for reexamining our approach to the treatment of pneumonia and for urgent research efforts proportional to the actual public health impact of the disease [18, 22].
Dysregulated Systemic Inflammation: A Biologic Rationale for the Use of Prolonged Low Doses of Corticosteroids in Patients with CAP
Systemic Inflammation and Pneumonia
There are two major components of an infection: pathogen(s) and the host inflammatory response. Pneumonia develops when pathogens invading the sterile lower respiratory tract activate the innate immune response to produce local and systemic inflammation [23, 24]. Even when the pulmonary inflammatory response is compartimentalized to the affected lung, patients admitted to the hospital with CAP or health-care-associated pneumonia (HCAP) have increased circulating levels of inflammatory and hemostasic markers (systemic inflammation) [17, 25••, 26, 27]. Irrespective of microbiological etiology, the host’s inability to fully downregulate systemic inflammation (i.e., dysregulated systemic inflammation) is the dominant pathogenetic process contributing to acute and long-term morbidity and mortality in CAP.
Persistent, as opposed to short-lived, elevation of circulating inflammatory and hemostasic markers over time strongly correlates with worsened hospital and 1-year mortality, independently of demographic characteristics and comorbidities.
The Role of Glucorticoid During Insufficient Adrenal Response and as Regulator of Inflammation
An intact hypothalamic–pituitary–adrenal (HPA) axis with an effective intracellular glucocorticoid (GC)-mediated antiinflammatory activity is indispensable for host survival during the stress response following exposure to an infectious agent. GCs are the most important physiologic inhibitors of inflammation [28] and affect thousands of genes involved in stress-related homeostasis [28, 29]. Both the physiological and pharmacological actions of GCs are mediated by the GC receptor (GR), a member of the nuclear receptor superfamily of ligand-dependent transcription factors. The GR enhances or represses transcription of target genes by binding specific elements of DNA and recruiting coactivators or corepressors that modulate the activity of RNA polymerase II by a unique means of cross-talk between nuclear and cell surface receptors [30]. Strong experimental and clinical evidence demonstrates that innate or treatment-induced reduction (downregulation) of systemic inflammation is necessary to decrease morbidity and improve survival in sepsis [31]. In patients with severe CAP and septic shock, a relatively insufficient adrenal response has been observed during infection, associated with a higher risk of death. The spectrum of this insufficient adrenal response has been shown in severe CAP, ALI/ARDS, severe sepsis, and whenever there is a GC tissue resistance together with an exaggerated and protracted proinflammatory response [32, 33]. The term critical illness related corticosteroid insufficiency (CIRCI) was recently proposed by an expert panel to describe the dysfunction of the HPA axis that occurs during the continuum of sepsis-associated systemic inflammation and other critical illnesses [34]. CIRCI can be defined as inadequate intracellular GC anti-inflammatory activity for the severity of the patient’s illness. The mechanisms leading to impaired GR-mediated downregulation of inflammation are complex and partly understood. In the simplest terms, CIRCI can result either from insufficient availability of GCs to the cell or from intracellular resistance/insensivity to GCs (despite elevated circulating cortisol) [34]. These two conditions are affected to a significant extent by the intensity of systemic inflammation and are potentially reversible with quantitatively and temporally adequate prolonged GC administration [35, 36].
There is consistent evidence that prolonged low doses of GCs in patients with severe sepsis and ARDS, mostly caused by pneumonia [37], downregulate systemic inflammation and significantly improve cardiovascular and pulmonary organ physiology [34, 35, 38].
Duration of Glucocorticoid Treatment
Duration of GC treatment is an important determinant of both efficacy and toxicity [39]. Optimization of therapy with GCs is affected by two factors: (1) biological duration of the disease process (systemic inflammation and CIRCI) and (2) recovery time of the HPA axis after treatment is discontinued [40].
1. Longitudinal measurements of plasma cytokine levels in CAP have shown that after clinical resolution of pneumonia, inflammatory cytokines (tumor necrosis factor [TNF]-α and interleukin [IL]-6) and D-dimer remain elevated for weeks [25••, 26]. While the clinical signs for systemic inflammatory response syndrome (SIRS: fever, tachycardia, tachypnea) tend to resolve within 3 days of admission [41], TNF-α and IL-6 remain elevated for at least 22 days (limit of measurement) [25••]. These data, similar to those reported for patients with sepsis-induced ARDS (most of whom had CAP) [42], clearly demonstrate that biological resolution lags weeks behind clinical resolution.
2. Prolonged GC treatment is associated with downregulation of GR levels and suppression of the HPA axis, affecting systemic inflammation after treatment is discontinued. Experimental work has shown that short-term exposure of alveolar macrophages [43] or animals to dexamethasone is followed by enhanced inflammatory cytokine response to endotoxin [44]. Similarly, normal human subjects pretreated with hydrocortisone had significantly higher TNF-α and IL-6 response after endotoxin challenge, as compared with controls [45]. GC treatment downregulates GR levels in most cell types, thereby decreasing the efficacy of the treatment. Downregulation occurs at both the transcriptional and traslational levels, and GC treatment decreases receptor half-life by approximately 50% [46]. In experimental animals, overexpression of GRs improves resistance to endotoxin-mediated septic shock, while GR blockade increases mortality [47]. Second, even after a few days of GC treatment, removal without tapering leads to adrenal suppression in 45% of patients, with gradual recovery over a period of 14 days [48]. The concept of rebound 24–36 h following removal of steroid treatment was initially reported by Wagner and collaborators in 1956 [49]. For decades, treatment-associated adrenal suppression has been part of standard teaching in medical schools; the product insert provided by the manufacturer (Pfizer) states that “drug-induced secondary adrenocortical insufficiency may be minimized by gradual reduction of dosage” [50].
Support for an association between longer duration of treatment and improved medium-term survival is provided by the Pneumocystis jiroveci pneumonia literature, where a 21-day treatment led to a sizable reduction in 3-month mortality (18% vs. 27%; p = .02) [51]. Another factor stressing the importance of duration of GC treatment is the actual biological duration of the disease process (systemic inflammation) that causes long-term morbidity and mortality [52].
The Long-Term Effects of Dysregulated Systemic Inflammation
An inflammatory and procoagulant load in patients who have pneumonia leads to excess morbidity and mortality both during hospitalization and after hospital discharge. The traditional pathophysiological model of pneumonia that equated the presence of SIRS with the duration of biologically significant systemic inflammation, which is damaging to the host, is incorrect. Biological resolution lags weeks behind clinical resolution; therefore, it is not a reliable indicator of disease activity. Pneumonia patients, admitted with or without severe sepsis, are discharged from the hospital with a clinically silent low-grade systemic inflammation and prothrombotic state that has a negative impact on mortality that is greater than one of acute (clinically apparent) systemic inflammation. The most robust contribution to this field originates from Kellum [25••] and Yende [16, 27], using the large GenIMS data set (1,886 CAP patients) that included daily measurements until day 7 and once weekly thereafter. Similar to inflammatory cytokynes, higher levels of D-dimer (hemostasis marker) at hospital admission correlated with worsened mortality in the hospital [43] and at 90 days [26]. Even after clinical resolution of pneumonia, inflammatory cytokynes (TNF-α and IL-6) and D-dimer remain elevated for weeks [25••, 26]. While the clinical signs for SIRS (fever, tachycardia, tachypnea) tend to resolve within 3 days of admission [42], TNF-α and IL-6 remain elevated for at least 22 days [25••]. IL-6 levels obtained at hospital discharge in clinically stable patients (>90% without SIRS criteria) strongly correlate with subsequent 1-year mortality after adjusting for age, race, gender, comorbidity score, and APACHE III [16]. The excess mortality in patients with low versus high IL-6 approximates the one reported by Kaplan et al. [7]. Similarly, higher levels of D-dimer at hospital discharge correlate with subsequent 1-year mortality and late cardiovascular events (myocardial infarction, stroke, and atherosclerotic heart disease) [27]. Table 1 shows the short- and long-term morbidity associated with acute and chronic systemic inflammation in patients with pneumonia. Particularly, a growing body of literature points to an association between systemic inflammation accompanying pneumonia and progression of underlying cardiovascular disease [45, 46]. Lowering this inflammatory load may improve short- (hospitalization) and long-term (post hospitalization) outcome. Unfortunately, all published randomized trials on GC in pneumonia and sepsis were designed without awareness of these fundamental concepts, so treatment of sepsis omitted being directed to dysregulated chronic inflammation and continued until disease resolution.
Steroids for CAP: Evidence from the Literature
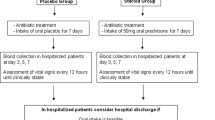
As early as 1956, favorable clinical effects of hydrocortisone (80 mg per day orally tapered over 5 days) were reported in patients with pneumococcal pneumonia [49]. However, after the introduction of antibiotics, research interest faded until recently. The use of GC treatment in order to modulate inflammation in patients with CAP was targeted in experimental studies and a few randomized clinical trials, but the results were not univocal. Moreover, in spite of a pathophysiologic rationale [53] for GC use in CAP only for a prolonged low dose (stress dose), most studies did not use this dose regimen.
Prolonged Low Dose of Glucocorticoids Covering 24 h/Day
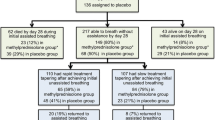
It is noticeable that only three recent randomized clinical trials (RCTs) [54•, 55, 56] were performed using a 24-h GC coverage with a prolonged low dose of GCs for at least 7 days in patients with CAP (Table 2). The other RCTs [57–62] used corticosteroids for a shorter number of days with or without a dose regimen covering 24 h/day (Table 3). It has been reported [63] that a longer duration of GC treatment covering 24 h/day may prevent rebound systemic inflammation and clinical deterioration due to abrupt discontinuation of GC treatment [64]. Not surprisingly, all the RCTs using a prolonged low dose of corticosteroids were able to reach the study end points. Otherwise, in the other group of RCTs, only the study by Meijvis [62], using a long-lasting corticosteroids (dexamethasone) for 4 days, got the primary end point reducing the length of hospitalization in comparison with placebo.
Experimental Literature
Two recent experimental studies [65, 66] have demonstrated that treatment with GCs, in comparison with no treatment, was associated with a significant reduction in circulating and pulmonary inflammatory cytokine levels [65, 66], an improvement in histopathological severity scores [65, 66], and a decreased pulmonary bacterial burden [65]. A large experimental study of Escherichia coli pneumonia in mice found that GC treatment effectively reduced the risk of death following challenge with low or high numbers of organisms [28].
Clinical Trials of Glucocorticoids for CAP
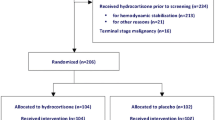
In the last 10 years, randomized controlled trials have investigated the effects of prolonged GC treatment in each phase of the temporal continuum of pneumonia-associated systemic inflammation: severe sepsis, septic shock, and ARDS. In these clinical trials, the protocol design varied widely for treatment (type of GC, daily dosage, duration of treatment, and tapering), patient selection, and study end points. Table 4 shows GC trials in patients with pneumonia-associated sepsis, severe sepsis, and ARDS [54•, 55–63, 67, 68]. Recent meta-analyses provided evidence of improvement in patient-centered outcomes for patients with septic shock [29] or ARDS, with most cases attributable to pneumonia [69, 70]. A limited number of trials have investigated GC treatment in patients with nonsevere [49, 56, 59–62] and severe [54•, 55, 58, 63, 69–71] pneumonia. Prospective, randomized clinical studies have also been undertaken, as follows.
-
1.
Severe pneumonia requiring ICU admission. Preliminary small trials investigating steroid treatment for at least 7 days duration [54•, 55] and subgroup analysis of trials in patients with severe sepsis [63], septic shock [67, 71], or ARDS [68] show weak evidence for a mortality benefit in patients with severe pneumonia requiring ICU admission (CAP/HCAP subgroup with high mortality). The two RCTs that investigated corticosteroid treatment exclusively in patients with pneumonia [54•, 55] consistently reported by day 8 a significant reduction in C-reactive protein level, duration of mechanical ventilation, organ dysfunction score, chest radiograph score, and development of septic shock. The most beneficial effect was in those patients on mechanical ventilation, both invasive and noninvasive. One of our trials [54•] found a reduction in mortality. A large Cooperative Study Program randomized trial (CSP #574 ESCAPe) is in progress to study the effect of prolonged treatment (20 days) with methylprednisolone in patients on mechanical ventilation (CSP #574 ESCAPe, ClinicalTrials.gov Identifier: NCT01283009).
-
2.
Nonsevere pneumonia. The literature for patients with nonsevere pneumonia includes six trials (n = 829; range, 56–304) conducted over a span of 54 years [49, 56, 59–62]. Three trials [56, 61, 62] were published over the last 15 months, underlying the intense interest in this field. These studies included patients with low acute mortality risk (6%) and were insufficiently powered to demonstrate a short-term mortality benefit. The largest trial [62] reported a significant reduction in duration of hospitalization (primary end point: 6.5 vs. 7.5 days; p < .05) and improved social functioning by day 30 (p < .01) without increased rate of adverse events. All but one trial [61] reported faster clinical resolution [49, 56, 59, 62]. No study addressed the effects of GC treatment on long-term morbidity and mortality.
Meta-Analysis of Glucorticoid Treatment for CAP
A meta-analysis by Nie et al. was published in 2012 [72] that included both randomized and quasirandomized trials of corticosteroid treatment in adult patients with CAP of mixed severity. The durations of different corticosteroid treatment ranged from 1 to 9 days. Effects on primary outcome (mortality) and secondary outcomes (adverse events) were accessed in this meta-analysis. Nine trials involving 1,001 patients were included [54•, 55–62]. Use of corticosteroids did not significantly reduce mortality when all studies were included (Peto odds ratio [OR] 0.62, 95% confidence interval [CI] 0.37–1.04; p = .07). In a subgroup analysis categorizing patients by severity, a survival benefit was found among severe CAP patients (Peto OR 0.26, 95% CI 0.11–0.64; p = .003). Importantly, a subgroup analysis by duration of corticosteroid treatment, significantly reduced mortality among patients with prolonged (>5 days) corticosteroid treatment (Peto OR 0.51, 95% CI 0.26–0.97; p = .04; I2 = 37%). Corticosteroids increased the risk of hyperglycemia (Peto OR 2.64, 95% CI 1.68–4.15; p = .0001), but without increasing the risk of gastroduodenal bleeding (Peto OR 1.67, 95% CI 0.41–6.80; p = .47) and superinfection (Peto OR 1.36, 95% CI 0.65–2.84; p = .41) [72]. We have performed a new meta-analysis (Fig. 1) based on the expanded literature shown in Table 4. This new analysis provides additional evidence for a significant short-term survival benefit in patients with severe CAP (p = .001). Figure 1 shows the Forrest plot of survival in patients with nonsevere CAP versus severe CAP.
Conclusions
The two major components of pneumonia are pathogen(s) and inflammation. Following the reduction in mortality associated with the introduction of antibiotics, morbidity and mortality for pneumonia has remained unchanged or increased. The greatest shift in our understanding of the true impact of pneumonia on the host has been the documentation of substantial continuing excess mortality for more than 2 years after surviving an episode of CAP. It is now appreciated that the degree of pneumonia-associated systemic inflammation at hospital presentation and at hospital discharge significantly contributes to acute and long-term morbidity and mortality more than do demographics and comorbidities [52]. For the foreseeable future, GCs will remain the most viable candidate for first-line treatment, thanks to their rapid and profound anti-inflammatory effect, safety profile, and low cost. The immunoregulatory effect of GCs given at a prolonged low-dose regimen is able to downregulate proinflammatory cytokyne production and to improve organ function. Nevertheless, the literature of GC treatment for pneumonia had controversial results due to different patient selection criteria, GC dose regimens, and study end points. However, only RCTs using a dose regimen of corticosteroids covering 24 h/day reached the study end points. A recent meta-analysis [72] of pooled controlled small trials showed improvement in acute mortality in patients with severe CAP, but not with CAP without severe sepsis [73]. We have expanded on this meta-analysis by including patients with CAP recruited in trials investigating prolonged low-dose GC treatment in septic shock and/or early acute respiratory distress syndrome. Our findings confirm a survival advantage for severe CAP. A large randomized trial is in progress to confirm the aggregate findings of these small trials and to evaluate the long-term effect of this low-cost treatment.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Armstrong GL, Conn LA, Pinner RW. Trends in infectious disease mortality in the United States during the 20th century. JAMA. 1999;281:61–6.
Heron MP, Hoyert DL, Murphy SL, Xu J, Kochanek KD, Tejada-Vera B. Deaths: final data for 2006. National Vital Statistics Reports; Vol 57, No 14. Hyattsville, MD: National Center for Health Statistics; 2009. http://www.cdc.gov/nchs/data/nvsr/nvsr60/nvsr60_03.pdf Accessed November 26, 2012.
Musher DM, Montoya R, Wanahita A. Diagnostic value of microscopic examination of Gram-stained sputum and sputum cultures in patients with bacteremic pneumococcal pneumonia. Clin Infect Dis. 2004;39:165–9.
Corrales-Medina VF, Musher DM. Immunomodulatory agents in the treatment of community-acquired pneumonia: a systematic review. J Infect. 2011;63:187–99.
LaCroix AZ, Lipson S, Miles TP, White L. Prospective study of pneumonia hospitalizations and mortality of U.S. older people: the role of chronic conditions, health behaviors, and nutritional status. Public Health Rep. 1989;104:350–60.
Brancati FL, Chow JW, Wagener MM, Vacarello SJ, Yu VL. Is pneumonia really the old man’s friend? Two-year prognosis after community-acquired pneumonia. Lancet. 1993;342:30–3.
Koivula I, Sten M, Makela PH. Prognosis after community-acquired pneumonia in the elderly: a population-based 12-year follow-up study. Arch Intern Med. 1999;159:1550–5.
Kaplan V, Clermont G, Griffin MF, et al. Pneumonia: still the old man’s friend? Arch Intern Med. 2003;163:317–23.
Hedlund JU, Ortqvist AB, Kalin ME, Granath F. Factors of importance for the long term prognosis after hospital treated pneumonia. Thorax. 1993;48:785–9.
Carriere KC, Jin Y, Marrie TJ, Predy G, Johnson DH. Outcomes and costs among seniors requiring hospitalization for community-acquired pneumonia in Alberta. J Am Geriatr Soc. 2004;52:31–8.
Waterer GW, Kessler LA, Wunderink RG. Medium-term survival after hospitalization with community-acquired pneumonia. Am J Respir Crit Care Med. 2004;169:910–4.
Bordon J, Wiemken T, Peyrani P, et al. Decrease in long-term survival for hospitalized patients with community-acquired pneumonia. Chest. 2010;138:279–83.
•• Yende S, D'angelo G, Kellum JA, et al. Inflammatory markers at hospital discharge predict subsequent mortality after pneumonia and sepsis. Am J Respir Crit Care Med. 2008;177:1242–7. Landmark study reporting that increased concentrations of tumour necrosis factor (TNF) α and interleukin 6 persisted for weeks after clinical resolution of pneumonia, and that the increase in interleukin 6 at discharge predicted subsequent 90-day and 1-year mortality.
Johnstone J, Eurich DT, Majumdar SR, Jin Y, Marrie TJ. Long-term morbidity and mortality after hospitalization with community-acquired pneumonia: a population-based cohort study. Medicine. 2008;87:329–34.
Capelastegui A, Espana Yandiola PP, Quintana JM, et al. Predictors of short-term rehospitalization following discharge of patients hospitalized with community-acquired pneumonia. Chest. 2009;136:1079–85.
El Solh A, Pineda L, Bouquin P, Mankowski C. Determinants of short and long term functional recovery after hospitalization for community-acquired pneumonia in the elderly: role of inflammatory markers. BMC Geriatr. 2006;6:12.
Yende S, D’Angelo G, Kellum JA, et al. Inflammatory markers at hospital discharge predict subsequent mortality after pneumonia and sepsis. Am J Respir Crit Care Med. 2008;177:1242–7.
Waterer GW, Rello J, Wunderink RG. Management of Community-acquired Pneumonia in Adults. Am J Respir Crit Care Med. 2011;183:157–64.
Iwashyna TJ, Ely EW, Smith DM, Langa KM. Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA. 2010;304:1787–94.
Centers for Medicare & Medicaid Services. CMS/ORDI, December 2008. Table V.4, Medicare Short-Stay Hospital DRGs Ranked by Discharges, Fiscal Year 2007. http://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/DataCompendium/16_2008DataCompendium.html Accessed December 3, 2012.
Hsu JL, Siroka AM, Smith MW, Holodniy M, Meduri GU. One-year outcomes of community-acquired and health-care-associated pneumonia in the Veterans Affairs Health Care System. Int J Infect Dis. 2011;15:e382–e7.
Mizgerd JP. Acute lower respiratory tract infection. N Engl J Med. 2008;358:716–27.
Englert JA, Fink MP. The Multiple Organ Dysfunction Syndrome and Late-phase Mortality in Sepsis. Curr Infect Dis Rep. 2005;7:335–41.
Dehoux MS, Boutten A, Ostinelli J, et al. Compartmentalized cytokine production within the human lung in unilateral pneumonia. Am J Respir Crit Care Med. 1994;150:710–6.
•• Kellum JA, Kong L, Fink MP, et al. Understanding the inflammatory cytokine response in pneumonia and sepsis: results of the Genetic and Inflammatory Markers of Sepsis (GenIMS) Study. Arch Intern Med. 2007;167:1655–63. Report from a large dataset including nearly 1900 patients with CAP (the Genetic and Inflammatory Markers of Sepsis Study) showing a new pathophysiological model based on prolonged inflammation. According to this model the duration of glucocorticoid treatment directed at achieving clinical resolution should be deemed inadequate.
Milbrandt EB, Reade MC, Lee M, et al. Prevalence and significance of coagulation abnormalities in community-acquired pneumonia. Mol Med. 2009;15:438–45.
Yende S, D’Angelo G, Mayr F, et al. Elevated hemostasis markers after pneumonia increases one-year risk of all cause and cardiovascular deaths. PLoS One. 2011;6:e22847.
Li Y, Cui X, Li X, et al. Risk of death does not alter the efficacy of hydrocortisone therapy in a mouse E. coli pneumonia model: Risk and corticosteroids in sepsis. Intensive Care Med. 2007;34:568–77.
Annane D, Bellissant E, Bollaert PE, et al. Corticosteroids in the treatment of severe sepsis and septic shock in adults: a systematic review. JAMA. 2009;301:2362–75.
Oakley RH, Revollo J, Cidlowsi JA. Glucocorticoids regulate arrestin gene expression and redirect the signaling profile of G-protein coupled receptors. PNAS. 2012;109:17591–6.
Meduri GU, Yates CR. Systemic inflammation-associated glucocorticoid resistance and outcome of ARDS. Ann NY Acad Sci. 2004;1024:24–53.
Salluh JI, Bozza FA, Soares M, et al. Adrenal response in severe community-acquired pneumonia: impact on outcomes and disease severity. Chest. 2008;134:947–54.
Meduri GU, Muthiah MP, Carratu P, Eltorky M, Chrousos GP. Nuclear factor-kappaB- and glucocorticoid receptor alpha- mediated mechanisms in the regulation of systemic and pulmonary inflammation during sepsis and acute respiratory distress syndrome. Evidence for inflammation-induced target tissue resistance to glucocorticoids. Neuroimmunomodulation. 2005;12:321–38.
Marik PE, Pastores S, Annane D, et al. Clinical practice guidelines for the diagnosis and management of corticosteroid insufficiency in critical illness: Reccomendations of an international task force. Crit Care Med. 2008;36:1937–49.
Meduri GU, Tolley EA, Chrousos GP, Stentz F. Prolonged methylprednisolone treatment suppresses systemic inflammation in patients with unresolving acute respiratory distress syndrome. Evidence for inadequate endogenous glucocorticoid secretion and inflammation-induced immune cell resistance to glucocorticoids. Am J Respir Crit Care Med. 2002;165:983–91.
Nakamori Y, Ogura H, Koh T, et al. The balance between expression of intranuclear NF-kappaB and glucocorticoid receptor in polymorphonuclear leukocytes in SIRS patients. J Trauma. 2005;59:308–14. discussion 14–15.
Laterre PF, Garber G, Levy H, et al. Severe community-acquired pneumonia as a cause of severe sepsis: Data from the PROWESS study. Crit Care Med. 2005;33:952–61.
Meduri GU, Annane D, Chrousos GP, Marik PE, Sinclair SE. Activation and regulation of systemic inflammation in ARDS: rationale for prolonged glucocorticoid therapy. Chest. 2009;136:1631–43.
Meduri GU. An historical review of glucocorticoid treatment in sepsis. Disease pathophysiology and the design of treatment investigation. Sepsis. 1999;3:21–38.
Briegel J, Jochum M, Gippner-Steppert C, Thiel M. Immunomodulation in Septic Shock: Hydrocortisone Differentially Regulates Cytokine Responses. J Am Soc Nephrol. 2001;12:S70–4.
Halm EA, Fine MJ, Marrie TJ, et al. Time to clinical stability in patients hospitalized with community-acquired pneumonia: implications for practice guidelines. JAMA. 1998;279:1452–7.
Meduri GU, Bell W, Sinclair S, Annane D. Pathophysiology of acute respiratory distress syndrome. Glucocorticoid receptor-mediated regulation of inflammation and response to prolonged glucocorticoid treatment. Presse Med. 2011;40:e543–e60.
Broug-Holub EKG. Dose- and time-dependent activation of rat alveolar macrophages by glucocorticoids. Clin Exp Immunol. 1996;104:332–6.
Fantuzzi G, Demitri MT, Ghezzi P. Differential effect of glucocorticoids on tumor necrosis factor production in mice: up-regulation by early pretreatment with dexamethasone. Clin Exp Immunol. 1994;96:166–9.
Barber AE, Coyle SM, Marano MA, et al. Glucocorticoid therapy alters hormonal and cytokine responses to endotoxin in man. J Immunol. 1993;150:1999–2006.
Schaaf MJ, Cidlowski JA. Molecular mechanisms of glucocorticoid action and resistance. J Steroid Biochem Mol Biol. 2002;83:37–48.
Cooper MS, Stewart PM. Adrenal insufficiency in critical illness. J Intensive Care Med. 2007;22:348–62.
Henzen C, Suter A, Lerch E, Urbinelli R, Schorno XH, Briner VA. Suppression and recovery of adrenal response after short-term, high-dose glucocorticoid treatment. Lancet. 2000;355:542–5.
Wagner HNJ, Bennett ILJ, Lasagna L, Cluff LE, Rosenthal MB, Mirick GS. The effect of hydrocortisone upon the course of pneumococcal pneumonia treated with penicillin. Bull Johns Hopkins Hosp. 1956;98:197–215.
Solu-medrol. Methylprednisolone sodium succinate product information. http://labeling.pfizer.com/ShowLabeling.aspx?id=648 Accessed December 3, 2012.
Briel M, Bucher HC, Boscacci R, et al. Adjunctive corticosteroids for Pneumocystis jiroveci pneumonia in patients with HIV- infection. Cochrane Database Syst Rev. 2006;3:CD006150.
Confalonieri M, Meduri GU. Glucocorticoid treatment in community-acquired pneumonia. Lancet. 2011;377:1982–4.
Galon J, Franchimont D, Hiroi N, et al. Gene profiling reveals unknown enhancing and suppressive actions of glucocorticoids on immune cells. FASEB J. 2002;16:61–71.
• Confalonieri M, Urbino R, Potena A, et al. Hydrocortisone infusion for severe community-acquired pneumonia: a preliminary randomized study. Am J Respir Crit Care Med. 2005;171:242–8. The first RCT on prolonged stress doses of corticosteroids in severe CAP showing beneficial effects on oxygenation, systemic inflammation, prevention of severe sepsis, and survival.
Sabry NA, Omar EE-D. Corticosteroids and ICU course of community acquired pneumonia in Egyptian settings. Pharmacology & Pharmacy. 2011;2:73–81.
Fernandez-Serrano S, Dorca J, Garcia-Vidal C, et al. Effect of corticosteroids on the clinical course of community-acquired pneumonia: a randomized controlled trial. Crit Care. 2011;15:R96.
van Woensel JB, van Aalderen WM, de Weerd W, et al. Dexamethasone for treatment of patients mechanically ventilated for lower respiratory tract infection caused by respiratory syncytial virus. Thorax. 2003;58:383–7.
Marik P, Kraus P, Sribante J, Havlik I, Lipman J, Johnson DW. Hydrocortisone and tumor necrosis factor in severe community-acquired pneumonia: a randomized controlled study. Chest. 1993;104:389–92.
McHardy VU, Schonell ME. Ampicillin dosage and use of prednisolone in treatment of pneumonia: co- operative controlled trial. Br Med J. 1972;4:569–73.
Mikami K, Suzuki M, Kitagawa H, et al. Efficacy of corticosteroids in the treatment of community-acquired pneumonia requiring hospitalization. Lung. 2007;185:249–55.
Snijders D, Daniels JM, de Graaff CS, van der Werf TS, Boersma WG. Efficacy of corticosteroids in community-acquired pneumonia - a randomized double blinded clinical trial. Am J Respir Crit Care Med. 2010;181:975–82.
Meijvis SC, Hardeman H, Remmelts HH, et al. Dexamethasone and length of hospital stay in patients with community-acquired pneumonia: a randomised, double-blind, placebo-controlled trial. Lancet. 2011;377:2023–30.
Nawab QU, Golden E, Confalonieri M, Umberger R, Meduri GU. Corticosteroid treatment in severe community-acquired pneumonia: duration of treatment affects control of systemic inflammation and clinical improvement. Intensive Care Med 2011; 37: 1553–4.
Keh D, Boehnke T, Weber-Cartens S, et al. Immunologic and hemodynamic effects of “low-dose” hydrocortisone in septic shock: a double-blind, randomized, placebo-controlled, cross- over study. Am J Respir Crit Care Med. 2003;167:512–20.
Sibila O, Luna CM, Agusti C, et al. Effects of glucocorticoids in ventilated piglets with severe pneumonia. Eur Respir J. 2008;32:1037–46.
Tagliabue C, Salvatore CM, Techasaensiri C, et al. The impact of steroids given with macrolide therapy on experimental Mycoplasma pneumoniae respiratory infection. J Infect Dis. 2008;198:1180–8.
Annane D, Sebille V, Charpentier C, et al. Effect of treatment with low doses of hydrocortisone and fludrocortisone on mortality in patients with septic shock. Jama. 2002;288:862–71.
Meduri GU, Golden E, Freire AX, et al. Methylprednisolone infusion in early severe ARDS: results of a randomized controlled trial. Chest. 2007;131:954–63.
Tang B, Craig J, Eslick G, Seppelt I, McLean A. Use of corticosteroids in acute lung injury and acute respiratory distress syndrome: a systematic review and meta-analysis. Crit Care Med. 2009;37:1594–603.
Meduri GU, Rocco PR, Annane D, Sinclair SE. Prolonged glucocorticoid treatment and secondary prevention in acute respiratory distress syndrome. Expert Rev Respir Med. 2010;4:201–10.
Sprung CL, Annane D, Keh D, et al. Hydrocortisone therapy for patients with septic shock. N Engl J Med. 2008;358:111–24.
Nie W, Zheng Y, Cheng J, Xiu Q. Corticosteroids in the treatment of community-acquired pneumonia in adults: a meta-analysis. PLoS ONE 7(10): e47926. doi:10.1371/journal.pone.0047926
Meduri GU, Bell WA, Confalonieri M. Glucocorticoid Treatment in Community-acquired Pneumonia without Severe Sepsis: No Evidence of Efficacy. Am J Respir Crit Care Med. 2010;181:880–1.
Disclosure
No potential conflicts of interest relevant to this article were reported.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Confalonieri, M., Annane, D., Antonaglia, C. et al. Is Prolonged Low-Dose Glucocorticoid Treatment Beneficial in Community-Acquired Pneumonia?. Curr Infect Dis Rep 15, 158–166 (2013). https://doi.org/10.1007/s11908-013-0322-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11908-013-0322-8