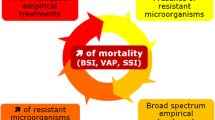
Abstract
In the 80s and 90s, increasing antibiotic resistance was met by the introduction of new effective agents with broader antibacterial spectra for the empirical treatment of severe infections. In recent years, however, few novel antimicrobials have been developed, and this has critically weakened our strength in the fight against resistant bacteria, especially Gram-negative bacilli. It has been well proven that mortality increases if initial empirical antibiotic treatment for severe infection is inappropriate due to resistance of the pathogen. Physicians are already faced with the increasing challenge of untreatable or almost untreatable Gram-negative infections due to antibiotic resistance. Empirical treatment with broader spectra and high antibiotic pressure both in- and outside hospital is the driving force behind resistance. Since new efficient drugs against Gram-negative bacilli will not be available for some time, the best we can do to stop infections caused by multidrug-resistant bacteria is to improve infection control and choice of antibiotics, which should be based on surveillance of local antibiotic consumption and resistance. We must learn more about the revived antibacterial agents colistin and fosfomycin, and the few next generation Gram-negative antibiotics that have been developed. The aim of this review is to give an update on present therapeutic options in the fight against multidrug-resistant Gram-negative bacteria.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
•• Cornaglia G, Giamarellou H, Rossolini GM. Metallo-[beta]-lactamases: a last frontier for [beta]-lactams? Lancet Infect Dis. 2011;11(5):381–93. doi:10.1016/s1473-3099(11)70056-1. A comprehensive and detailed review in which the problem of the worldwide spread of metallo-β-lactamases is analyzed being a major challenge both for treatment and for infection control policies.
• Hawkey PM, Jones AM. The changing epidemiology of resistance. J Antimicrob Chemother. 2009;64 Suppl 1:i3–10. doi:10.1093/jac/dkp256. A review of new mechanism of resistance and trends in global spread of MDR bacteria including ESBLs.
• Freire-Moran L, Aronsson B, Manz C, et al. Critical shortage of new antibiotics in development against multidrug-resistant bacteria—Time to react is now. Drug Resistance Updates. 2011;14(2):118–24. doi:10.1016/j.drup.2011.02.003. This study investigated the status of the antibacterial drug development pipeline of new agents that have entered clinical development.
Magiorakos A, Srinivasan A, Carey R, et al. Multidrug resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2011(Accepted Article). doi:10.1111/j.1469-0691.2011.03570.x.
• ECDC/EMEA Joint technical report. The bacterial challenge: time to react A call to narrow the gap between multidrug-resistant bacteria in the EU and the development of new antibacterial agents. 2009. http://ecdc.europa.eu/en/publications/Publications/0909_TER_The_Bacterial_Challenge_Time_to_React.pdf. Accessed June 10 2011. This report describes the gap between the burden of infections due to multidrug-resistant bacteria and the development of new antibiotics.
Doyle JS, Buising KL, Thursky KA, et al. Epidemiology of infections acquired in intensive care units. Semin Respir Crit Care Med. 2011;32(2):115–38. doi:10.1055/s-0031-1275525.
Goel N, Wattal C, Oberoi JK, et al. Trend analysis of antimicrobial consumption and development of resistance in non-fermenters in a tertiary care hospital in Delhi, India. J Antimicrob Chemother. 2011;66(7):1625–30. doi:10.1093/jac/dkr167.
• Souli M, Galani I, Giamarellou H. Emergence of extensively drug-resistant and pandrug-resistant Gram-negative bacilli in Europe. Euro Surveill. 2008;13(47). A review of mechanism of multidrug resistant of gram-negative bacteria, risk factors for emergence and prevalence in European countries and burden of resistance.
Abhilash KP, Veeraraghavan B, Abraham OC. Epidemiology and outcome of bacteremia caused by extended spectrum beta-lactamase (ESBL)-producing Escherichia coli and Klebsiella spp. in a tertiary care teaching hospital in south India. J Assoc Physicians India. 2010;58(Suppl):13–7.
Jabeen K, Zafar A, Irfan S, et al. Increase in isolation of extended spectrum beta lactamase producing multidrug resistant non typhoidal Salmonellae in Pakistan. BMC Infect Dis. 2010;10:101. doi:10.1186/1471-2334-10-101.
Ofner-Agostini M, Simor A, Mulvey M, et al. Risk factors for and outcomes associated with clinical isolates of Escherichia coli and Klebsiella species resistant to extended-spectrum cephalosporins among patients admitted to Canadian hospitals. Can J Infect Dis Med Microbiol. 2009;20(3):e43–8.
Uma B, Prabhakar K, Rajendran S, Lakshmi Sarayu Y. Prevalence of extended spectrum beta lactamases in Salmonella species isolated from patients with acute gastroenteritis. Indian J Gastroenterol. 2010;29(5):201–4. doi:10.1007/s12664-010-0044-x.
• Yu F, Chen Q, Yu X, et al. High prevalence of extended-spectrum beta lactamases among Salmonella enterica Typhimurium isolates from pediatric patients with diarrhea in China. PLoS One. 2011;6(3):e16801. doi:10.1371/journal.pone.0016801. The study demonstrates significant multidrug resistance among S.enterica typhimurium within a pediatric population in China, including a higher prevalence of broadspectrum cephalosporin resistance.
• Allegranzi B, Bagheri Nejad S, Combescure C, et al. Burden of endemic health-care-associated infection in developing countries: systematic review and meta-analysis. Lancet. 2011;377(9761):228–41. doi:10.1016/S0140-6736(10)61458-4. A comprehensive review and meta-analysis of burden of health care-associated infection in developing countries.
Johansson M, Phuong DM, Walther SM, Hanberger H. Need for improved antimicrobial and infection control stewardship in Vietnamese intensive care units. Trop Med Int Health. 2011;16(6):737–43. doi:10.1111/j.1365-3156.2011.02753.x.
Rosenthal VD. Health-care-associated infections in developing countries. Lancet. 2011;377(9761):186–8. doi:10.1016/s0140-6736(10)62005-3.
• Rosenthal VD, Maki DG, Jamulitrat S, et al. International Nosocomial Infection Control Consortium (INICC) report, data summary for 2003–2008, issued June 2009. Am J Infect Control. 2010;38:95–104.e2. doi:10.1016/j.ajic.2009.12.004. In this recent INICC report on device-associated health-care associated infection rates from 25 developing countries were substantially higher in INICC intensive-care units than CDC/NHSN rates from the USA.
Rosenthal VD, Maki DG, Salomao R, et al. Device-associated nosocomial infections in 55 intensive care units of 8 developing countries. Ann Intern Med. 2006;145(8):582–91.
Gagliotti C, Balode A, Baquero F, et al. Escherichia coli and Staphylococcus aureus: bad news and good news from the European Antimicrobial Resistance Surveillance Network (EARS-Net, formerly EARSS), 2002 to 2009. Euro Surveill. 2011;16(11).
Bonomo RA. New Delhi metallo-beta-lactamase and multidrug resistance: a global SOS? Clin Infect Dis. 2011;52(4):485–7. doi:10.1093/cid/ciq179.
•• Grundmann H, Livermore DM, Giske CG, et al. Carbapenem-non-susceptible Enterobacteriaceae in Europe: conclusions from a meeting of national experts. Euro Surveill. 2010;15(46). This report provides information on the epidemiologic situation of carbapenem-non-susceptible Enterobacteriaceae in European countries and actions needed to prevent the endemic establishment of carbapenemase-producing Enterobacteriaceae in European hospitals.
• Kumarasamy KK, Toleman MA, Walsh TR, et al. Emergence of a new antibiotic resistance mechanism in India, Pakistan, and the UK: a molecular, biological, and epidemiological study. Lancet Infect Dis. 2010;10(9):597–602. doi:10.1016/S1473-3099(10)70143-2. A report of molecular, biological, and epidemiological data on New Delhi metallo-β-lactamase 1 (NDM-1) positive Enterobacteriaceae in India and Pakistan and the importation of the resistance gene into the UK by patients returning from the Indian subcontinent.
Nseir S, Blazejewski C, Lubret R, et al. Risk of acquiring multidrug-resistant Gram-negative bacilli from prior room occupants in the intensive care unit. Clin Microbiol Infect. 2010. doi:10.1111/j.1469-0691.2010.03420.x.
Gastmeier P. Healthcare-associated versus community-acquired infections: a new challenge for science and society. Int J Med Microbiol. 2010;300(6):342–5. doi:10.1016/j.ijmm.2010.04.007.
• Bush K. Alarming [beta]-lactamase-mediated resistance in multidrug-resistant Enterobacteriaceae. Curr Opin Microbiol. 2010;13(5):558–64. doi:10.1016/j.mib.2010.09.006. A comprehensive review of b-lactam resistance in Gram-negative pathogens
Jacoby GA. AmpC beta-lactamases. Clin Microbiol Rev. 2009;22(1):161–82, Table of Contents. doi:10.1128/CMR.00036-08.
Sader HS, Hsiung A, Fritsche TR, Jones RN. Comparative activities of cefepime and piperacillin/tazobactam tested against a global collection of Escherichia coli and Klebsiella spp. with an ESBL phenotype. Diagn Microbiol Infect Dis. 2007;57(3):341–4. doi:10.1016/j.diagmicrobio.2006.08.016.
Lee K, Yong D, Choi YS, et al. Reduced imipenem susceptibility in Klebsiella pneumoniae clinical isolates with plasmid-mediated CMY-2 and DHA-1 beta-lactamases co-mediated by porin loss. Int J Antimicrob Agents. 2007;29(2):201–6. doi:10.1016/j.ijantimicag.2006.09.006.
• Bonomo RA, Szabo D. Mechanisms of multidrug resistance in Acinetobacter species and Pseudomonas aeruginosa. Clin Infect Dis. 2006;43 Suppl 2:S49–56. doi:10.1086/504477. This review describes the molecular basis for antibiotic resistance in Acinetobacter species and P. aeruginosa.
Walsh TR. Clinically significant carbapenemases: an update. Curr Opin Infect Dis. 2008;21(4):367–71. doi:10.1097/QCO.0b013e328303670b.
Ramirez MS, Tolmasky ME. Aminoglycoside modifying enzymes. Drug Resist Updat. 2010;13(6):151–71. doi:10.1016/j.drup.2010.08.003.
Zhou Y, Yu H, Guo Q, et al. Distribution of 16S rRNA methylases among different species of Gram-negative bacilli with high-level resistance to aminoglycosides. Eur J Clin Microbiol Infect Dis. 2010;29(11):1349–53. doi:10.1007/s10096-010-1004-1.
Piddock LJ. Mechanisms of fluoroquinolone resistance: an update 1994–1998. Drugs. 1999;58 Suppl 2:11–8.
Strahilevitz J, Jacoby GA, Hooper DC, Robicsek A. Plasmid-mediated quinolone resistance: a multifaceted threat. Clin Microbiol Rev. 2009;22(4):664–89. doi:10.1128/CMR.00016-09.
Jiang Y, Zhou Z, Qian Y, et al. Plasmid-mediated quinolone resistance determinants qnr and aac(6′)-Ib-cr in extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae in China. J Antimicrob Chemother. 2008;61(5):1003–6. doi:10.1093/jac/dkn063.
Boucher HW, Talbot GH, Bradley JS, Edwards JE, Gilbert D, Rice LB, et al. Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clin Infect Dis. 2009;48(1):1–12. doi:10.1086/595011.
•• Kumar A, Roberts D, Wood KE, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med. 2006;34(6):1589–96. doi:10.1097/01.CCM.0000217961.75225.E9. An extremely important study where it is shown that effective antimicrobial administration within the first hour of documented hypotension is associated with increased survival, whereas there-after each hour of delay over the ensuing 6 hours is associated with decreased survival of 7.6%.
Giamarellou H. Multidrug-resistant Gram-negative bacteria: how to treat and for how long. Int J Antimicrob Agents. 2010;36 Suppl 2:S50–4. doi:10.1016/j.ijantimicag.2010.11.014.
•• Souli M, Galani I, Antoniadou A, et al. An outbreak of infection due to beta-Lactamase Klebsiella pneumoniae Carbapenemase 2-producing K. pneumoniae in a Greek University Hospital: molecular characterization, epidemiology, and outcomes. Clin Infect Dis. 2010;50(3):364–73. doi:10.1086/649865. The emergence of KPC-2 positive K. pneumoniae creating the first outbreak in a Greek University Hospital is described in detail where the strain was rapidly disseminated by cross transmission in 38 patients. Most patients received a colistin-containing regimen. Attributable mortality was 22.2% in ICU and 33.3% in non-ICU patients respectively.
Li J, Nation RL. Old polymyxins are back: is resistance close? Clin Infect Dis. 2006;43(5):663–4. doi:10.1086/506571.
Walkty A, DeCorby M, Nichol K, et al. In vitro activity of colistin (polymyxin E) against 3,480 isolates of gram-negative bacilli obtained from patients in Canadian hospitals in the CANWARD study, 2007–2008. Antimicrob Agents Chemother. 2009;53(11):4924–6. doi:10.1128/AAC.00786-09.
Giamarellos-Bourboulis EJ, Sambatakou H, Galani I, Giamarellou H. In vitro interaction of colistin and rifampin on multidrug-resistant Pseudomonas aeruginosa. J Chemother. 2003;15(3):235–8.
• Souli M, Rekatsina PD, Chryssouli Z, et al. Does the activity of the combination of imipenem and colistin in vitro exceed the problem of resistance in metallo-beta-lactamase-producing Klebsiella pneumoniae isolates? Antimicrob Agents Chemother. 2009;53(5):2133–5. doi:10.1128/AAC.01271-08. In 42 K. pneumoniae strains VIM-1 positive, regardless of imipenem MIC, the combination was synergistic (50%) or indifferent (50%) against colistin-susceptible strains, but it was antagonistic (55.6%) against non-colistin-susceptible strains.
Giamarellou H, Poulakou G. Multidrug-resistant Gram-negative infections: what are the treatment options? Drugs. 2009;69(14):1879–901. doi:10.2165/11315690-000000000-00000.
• Hachem RY, Chemaly RF, Ahmar CA, et al.. Colistin is effective in treatment of infections caused by multidrug-resistant Pseudomonas aeruginosa in cancer patients. Antimicrob Agents Chemother. 2007;51(6):1905–11. doi:10.1128/AAC.01015-06. This is the largest retrospective study in the literature in which colistin is shown to be effective in cancer patients, also including cases with neutropenia.
• Kallel H, Hergafi L, Bahloul M, et al. Safety and efficacy of colistin compared with imipenem in the treatment of ventilator-associated pneumonia: a matched case-control study. Intensive Care Med. 2007;33(7):1162–7. doi:10.1007/s00134-007-0675-2. In this study, monotherapy with colistin was compared to imipenem monotherapy against XDR and MDR non-formatters strains, indicating, though retrospectively, that colistin monotherapy can be adequate in patients with VAP.
Rios FG, Luna CM, Maskin B, et al. Ventilator-associated pneumonia due to colistin susceptible-only microorganisms. Eur Respir J. 2007;30(2):307–13. doi:10.1183/09031936.00156906.
Garnacho-Montero J, Ortiz-Leyba C, Jimenez-Jimenez FJ, et al. Treatment of multidrug-resistant Acinetobacter baumannii ventilator-associated pneumonia (VAP) with intravenous colistin: a comparison with imipenem-susceptible VAP. Clin Infect Dis. 2003;36(9):1111–8. doi:10.1086/374337.
Paul M, Bishara J, Levcovich A, et al. Effectiveness and safety of colistin: prospective comparative cohort study. J Antimicrob Chemother. 2010;65(5):1019–27. doi:10.1093/jac/dkq069.
Reina R, Estenssoro E, Saenz G, et al. Safety and efficacy of colistin in Acinetobacter and Pseudomonas infections: a prospective cohort study. Intensive Care Med. 2005;31(8):1058–65. doi:10.1007/s00134-005-2691-4.
• Falagas ME, Rafailidis PI, Ioannidou E, et al. Colistin therapy for microbiologically documented multidrug-resistant Gram-negative bacterial infections: a retrospective cohort study of 258 patients. Int J Antimicrob Agents. 2010;35(2):194–9. doi:10.1016/j.ijantimicag.2009.10.005. A retrospective study in which the authors showed that for strains of P. aeruginosa and A. baumanni, colistin monotherapy is equally as effective as the combination of colistin with meropenem.
Meletis G, Tzampaz E, Sianou E, et al. Colistin heteroresistance in carbapenemase-producing Klebsiella pneumoniae. J Antimicrob Chemother. 2011;66(4):946–7. doi:10.1093/jac/dkr007.
Yau W, Owen RJ, Poudyal A, et al. Colistin hetero-resistance in multidrug-resistant Acinetobacter baumannii clinical isolates from the Western Pacific region in the SENTRY antimicrobial surveillance programme. J Infect. 2009;58(2):138–44. doi:10.1016/j.jinf.2008.11.002.
Korbila IP, Michalopoulos A, Rafailidis PI, et al. Inhaled colistin as adjunctive therapy to intravenous colistin for the treatment of microbiologically documented ventilator-associated pneumonia: a comparative cohort study. Clin Microbiol Infect. 2010;16(8):1230–6. doi:10.1111/j.1469-0691.2009.03040.x.
• Falagas ME, Bliziotis IA, Tam VH. Intraventricular or intrathecal use of polymyxins in patients with Gram-negative meningitis: a systematic review of the available evidence. Int J Antimicrob Agents. 2007;29(1):9–25. doi:10.1016/j.ijantimicag.2006.08.024. This review includes the largest number of patients in the literature in which intraventicular or intrathecal colistin was mode of therapy in Gram-negative meningitis.
•• Plachouras D, Karvanen M, Friberg LE, et al. Population pharmacokinetic analysis of colistin methanesulfonate and colistin after intravenous administration in critically ill patients with infections caused by gram-negative bacteria. Antimicrob Agents Chemother. 2009;53(8):3430–6. doi:10.1128/AAC.01361-08. This study, for the first time in the literature, proves after applying specific methodology, the real serum PKs of intravenous colistin (without measuring colistimethate), showing that colistin displays a prolonged half-life of 14.4h. Based on the low plasma colistin levels after the first dose, the question of the use of a loading dose was raised.
Alberto C, Mendes C, Burdmann EA. Polymyxins—a review focusing on their nephrotoxicity. Rev Assoc Med Bras. 2010;56:752–8.
Fernandez L, Gooderham WJ, Bains M, et al. Adaptive resistance to the “last hope” antibiotics polymyxin B and colistin in Pseudomonas aeruginosa is mediated by the novel two-component regulatory system ParR-ParS. Antimicrob Agents Chemother. 2010;54(8):3372–82. doi:10.1128/AAC.00242-10.
•• Antoniadou A, Kontopidou F, Poulakou G, et al. Colistin-resistant isolates of Klebsiella pneumoniae emerging in intensive care unit patients: first report of a multiclonal cluster. J Antimicrob Chemother. 2007;59(4):786–90. doi:10.1093/jac/dkl562. Selective pressure due to extensive or inadequate colistin use may lead to the emergence of colistin resistance among K. pneumoniae isolates, jeopardizing treatment options in the ICU, potentially increasing morbidity and mortality of critically ill patients and necessiting prudent use of colistin.
Marchaim D, Chopra T, Pogue JM, et al. Outbreak of colistin-resistant, carbapenem-resistant Klebsiella pneumoniae in metropolitan Detroit, Michigan. Antimicrob Agents Chemother. 2011;55(2):593–9. doi:10.1128/AAC.01020-10.
Giamarellou H. Colistin: the loss of the last frontier? APUA Newsletter. 2007;25:5.
Matthaiou DK, Michalopoulos A, Rafailidis PI, et al. Risk factors associated with the isolation of colistin-resistant gram-negative bacteria: a matched case-control study. Crit Care Med. 2008;36(3):807–11. doi:10.1097/CCM.0B013E3181652FAE.
Castanheira M, Sader HS, Deshpande LM, et al. Antimicrobial activities of tigecycline and other broad-spectrum antimicrobials tested against serine carbapenemase- and metallo-beta-lactamase-producing Enterobacteriaceae: report from the SENTRY Antimicrobial Surveillance Program. Antimicrob Agents Chemother. 2008;52(2):570–3. doi:10.1128/AAC.01114-07.
Souli M, Kontopidou FV, Koratzanis E, et al. In vitro activity of tigecycline against multiple-drug-resistant, including pan-resistant, gram-negative and gram-positive clinical isolates from Greek hospitals. Antimicrob Agents Chemother. 2006;50(9):3166–9. doi:10.1128/AAC.00322-06.
Poulakou G, Kontopidou FV, Paramythiotou E, et al. Tigecycline in the treatment of infections from multi-drug resistant gram-negative pathogens. J Infect. 2009;58(4):273–84. doi:10.1016/j.jinf.2009.02.009.
Cai Y, Wang R, Liang B, et al. Systematic review and meta-analysis of the effectiveness and safety of tigecycline for treatment of infectious disease. Antimicrob Agents Chemother. 2011;55(3):1162–72. doi:10.1128/AAC.01402-10.
Ellis-Grosse EJ, Babinchak T, Dartois N, et al. The efficacy and safety of tigecycline in the treatment of skin and skin-structure infections: results of 2 double-blind phase 3 comparison studies with vancomycin-aztreonam. Clin Infect Dis. 2005;41 Suppl 5:S341–53. doi:10.1086/431675.
FDA U.S. Food and Drug Administration. FDA Drug Safety Communication: Increased risk of death with Tygacil (tigecycline) compared to other antibiotics used to treat similar infections. 2010. http://www.fda.gov/Drugs/DrugSafety/ucm224370.htm. Accessed June 2 2011.
Peleg AY, Potoski BA, Rea R, et al. Acinetobacter baumannii bloodstream infection while receiving tigecycline: a cautionary report. J Antimicrob Chemother. 2007;59(1):128–31. doi:10.1093/jac/dkl441.
Falagas ME, Kastoris AC, Kapaskelis AM, Karageorgopoulos DE. Fosfomycin for the treatment of multidrug-resistant, including extended-spectrum [beta]-lactamase producing, Enterobacteriaceae infections: a systematic review. Lancet Infect Dis. 2010;10(1):43–50. doi:10.1016/s1473-3099(09)70325-1.
Livermore DM, Warner M, Mushtaq S, et al. What remains against carbapenem-resistant Enterobacteriaceae? Evaluation of chloramphenicol, ciprofloxacin, colistin, fosfomycin, minocycline, nitrofurantoin, temocillin and tigecycline. Int J Antimicrob Agents. 2011;37(5):415–9. doi:10.1016/j.ijantimicag.2011.01.012.
Falagas ME, Maraki S, Karageorgopoulos DE, et al. Antimicrobial susceptibility of multidrug-resistant (MDR) and extensively drug-resistant (XDR) Enterobacteriaceae isolates to fosfomycin. Int J Antimicrob Agents. 2010;35(3):240–3. doi:10.1016/j.ijantimicag.2009.10.019.
Endimiani A, Patel G, Hujer KM, et al. In vitro activity of fosfomycin against blaKPC-containing Klebsiella pneumoniae isolates, including those nonsusceptible to tigecycline and/or colistin. Antimicrob Agents Chemother. 2010;54(1):526–9. doi:10.1128/AAC.01235-09.
Falagas ME, Giannopoulou KP, Kokolakis GN, Rafailidis PI. Fosfomycin: use beyond urinary tract and gastrointestinal infections. Clin Infect Dis. 2008;46(7):1069–77. doi:10.1086/527442.
Michalopoulos A, Virtzili S, Rafailidis P, et al. Intravenous fosfomycin for the treatment of nosocomial infections caused by carbapenem-resistant Klebsiella pneumoniae in critically ill patients: a prospective evaluation. Clin Microbiol Infect. 2010;16(2):184–6. doi:10.1111/j.1469-0691.2009.02921.x.
• Daikos GL, Petrikkos P, Psichogiou M, et al. Prospective observational study of the impact of VIM-1 metallo-beta-lactamase on the outcome of patients with Klebsiella pneumoniae bloodstream infections. Antimicrob Agents Chemother. 2009;53(5):1868–73. doi:10.1128/AAC.00782-08. In 162 consecutive patients with K. pneumoniae bacteraemia the predictive value of an MIC ≤4μg/ml for VIM-1-positive strains indicate a combination of meropenem with another active antibiotic to lower mortality whereas the administration of one active drug was equal to no active drug.
• Crandon JL, Bulik CC, Nicolau DP. In vivo efficacy of 1- and 2-gram human simulated prolonged infusions of doripenem against Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2009;53(10):4352–6. doi:10.1128/AAC.00282-09. Advantageous PD target attainment of 1 and 2g doses of doripenem given as a 4h infusion in mice and humans even for P. aeruginosa strains with MIC 8-16μg/ml were observed.
Adams-Haduch JM, Potoski BA, Sidjabat HE, et al. Activity of temocillin against KPC-producing Klebsiella pneumoniae and Escherichia coli. Antimicrob Agents Chemother. 2009;53(6):2700–1. doi:10.1128/AAC.00290-09.
Levin AS, Levy CE, Manrique AE, et al. Severe nosocomial infections with imipenem-resistant Acinetobacter baumannii treated with ampicillin/sulbactam. Int J Antimicrob Agents. 2003;21(1):58–62.
Endimiani A, Hujer KM, Hujer AM, et al. ACHN-490, a neoglycoside with potent in vitro activity against multidrug-resistant Klebsiella pneumoniae isolates. Antimicrob Agents Chemother. 2009;53(10):4504–7. doi:10.1128/AAC.00556-09.
Bassetti M, Ginocchio F, Giacobbe DR, Mikulska M. Development of antibiotics for Gram-negatives: where now? Clin Investig. 2011;1:211–27. doi:10.4155/cli.10.31.
Carmeli Y, Akova M, Cornaglia G, et al. Controlling the spread of carbapenemase-producing Gram-negatives: therapeutic approach and infection control. Clin Microbiol Infect. 2010;16(2):102–11. doi:10.1111/j.1469-0691.2009.03115.x.
Pakyz AL, Oinonen M, Polk RE. Relationship of carbapenem restriction in 22 university teaching hospitals to carbapenem use and carbapenem-resistant Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2009;53(5):1983–6. doi:10.1128/AAC.01535-08.
Acknowledgement
Thanks to Martin Holmbom (research assistant) for his help in dealing with online resources.
Disclosure
No potential conflicts of interest relevant to this article were reported.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hanberger, H., Giske, C.G. & Giamarellou, H. When and How to Cover for Resistant Gram-Negative Bacilli in Severe Sepsis and Septic Shock. Curr Infect Dis Rep 13, 416–425 (2011). https://doi.org/10.1007/s11908-011-0200-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11908-011-0200-1