Abstract
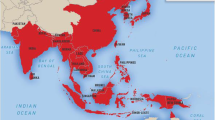
Epidemics of encephalitis occurring throughout much of Asia are caused by Japanese encephalitis virus (JEV), a flavivirus maintained in a zoonotic cycle and transmitted by the mosquito, Culex tritaeniorhynchus. Resident populations, including short–or long-term visitors to enzootic regions, are at risk for Japanese encephalitis (JE) infection and disease. For the past several decades, effective killed viral vaccines prepared in tissue culture or mouse brain have been used to immunize travelers and residents of affected countries. Cost, efficacy, and safety concerns led to the development of a single-dose live attenuated virus vaccine (SA14-14-2) and more recently, to the licensure in the United States, Europe, and Australia of a purified inactivated, tissue culture–based JE vaccine (IC51; Intercell AG, Vienna, Austria) and the soon-to-be-licensed live-attenuated yellow fever-JE chimeric vaccine (ChimeriVax-JE; Sanofi Pasteur, Lyon, France). Safe and effective JE vaccines are now available to the entire at-risk population and should greatly diminish the burden of disease.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance, •• Of major importance
Solomon T: Flavivirus encephalitis. N Engl J Med 2004, 351:370–378.
Halstead S, Jacobson J: Japanese encephalitis vaccines. In Vaccines, edn 5. Edited by Plotkin SA, Orenstein WA, Offit PA. Philadelphia: Elsevier; 2008:311–352.
Halstead SB, Grosz CR: Subclinical Japanese encephalitis. I. Infection of Americans with limited residence in Korea. Am J Hyg 1962, 75:190–201.
Benenson MW, Top FH Jr, Gresso W, et al.: The virulence to man of Japanese encephalitis virus in Thailand. Am J Trop Med Hyg 1975, 24(6 Pt 1):974–980.
Gajanana A, Thenmozhi V, Samuel PP, Reuben R: A community-based study of subclinical flavivirus infections in children in an area of Tamil Nadu, India, where Japanese encephalitis is endemic. Bull WHO 1995, 73: 237–244.
Solomon T, Vaughn DW: Pathogenesis and clinical features of Japanese encephalitis and West Nile virus infections. Curr Top Micro Immunol 2002, 267:171–194.
• Vaughn DW, Barrett A, Solomon T: Flaviviruses (yellow fever, dengue, dengue hemorrhagic fever, Japanese encephalitis, St. Louis encephalitis, tick-borne encephalitis). In Principals and Practice of Infectious Diseases, edn 7. Edited by Mandell GL, Bennett JE, Dolin R. Philadelphia: Churchill Livingstone; 2009:2133–2156. This chapter provides a comprehensive clinical overview of diseases caused by flaviviruses.
Solomon T, Dung NM, Kneen R, et al.: Seizures and raised intracranial pressure in Vietnamese patients with Japanese encephalitis. Brain 2002, 125(Pt 5):1084–1093.
Dickerson RB, Newton JR, Hansen JE: Diagnosis and immediate prognosis of Japanese B encephalitis; observations based on more than 200 patients with detailed analysis of 65 serologically confirmed cases. Am J Med 1952, 12: 277–88.
Kimura K, Dosaka A, Hashimoto Y, et al.: Single-photon emission CT findings in acute Japanese encephalitis. AJNR Am J Neuroradiol 1997, 18: 465–469.
Misra UK, Kalita J, Jain SK, et al.: Radiological and neurophysiological changes in Japanese encephalitis. J Neurol Neurosurg Psych 1994, 57: 1484–1487.
Kumar R, Mathur A, Singh KB, et al.: Clinical sequelae of Japanese encephalitis in children. Ind J Med Res 1993, 97:9–13.
Harinasuta C, Nimmanitya S, Titsyakorn U: The effect of interferon-alpha A on two cases of Japanese encephalitis in Thailand. Southeast Asian J Trop Med Public Health 1985, 16: 332–336.
Kumar R, Tripathi P, Baranwal M, et al.: Randomized, controlled trial of oral ribavirin for Japanese encephalitis in children in Uttar Pradesh, India. Clin Infect Dis 2009, 48:400–406.
Hammon WM, Tigertt WD, Sather GE, et al.: Epidemiologic studies of concurrent virgin epidemics of Japanese B encephalitis and of mumps on Guam, 1947–1948, with subsequent observations including dengue, through 1957. Am J Trop Med Hyg 1958, 7:441–467.
Libraty DH, Nisalak A, Endy TP, et al.: Clinical and immunological risk factors for severe disease in Japanese encephalitis. Trans R Soc Trop Med Hyg 2002, 96:173–178.
Luo D, Yao R, Song J, et al.: The effect of DDT spraying and bed nets impregnated with pyrethroid insecticide on the incidence of Japanese encephalitis virus infection. Trans R Soc Trop Med Hyg 1994, 88:629–631.
Luo D, Zhang K, Song J, et al.: The protective effect of bed nets impregnated with pyrethroid insecticide and vaccination against Japanese encephalitis. Trans R Soc Trop Med Hyg 1994, 88:632–634.
Markoff L: Points to consider in the development of a surrogate for efficacy of novel Japanese encephalitis virus vaccines. Vaccine 2000, 18(Suppl 2):26–32.
Hombach J, Solomon T, Kurane I, et al.: Report on a WHO consultation on immunological endpoints for evaluation of new Japanese encephalitis vaccines, WHO, Geneva, 2–3 September, 2004. Vaccine 2005, 23:5205–5211.
Paulke-Korinek M, Kollaritsch H: Japanese encephalitis and vaccines: past and future prospects. Wien Klin Wochenschr 2008, 120(19–20 Suppl 4):15–19.
•• Schioler KL, Samuel M, Wai KL: Vaccines for preventing Japanese encephalitis. Cochrane Database Syst Rev 2007. Available at http://www.cochrane.org/reviews/en/ab004263.html. Accessed February 2010. The authors performed an authoritative search of the literature through March 2007 for trials assessing Japanese encephalitis vaccine in terms of effectiveness, adverse events, and immunogenicity.
Jelinek T: Japanese encephalitis vaccine in travelers. Expert Rev Vaccines 2008, 7:689–693.
Inactivated Japanese encephalitis virus vaccine. Recommendations of the Advisory Committee on Immunization Practices (ACIP).MMWR Recomm Rep 1993, 42(RR-1):1–15.
Global Advisory Committee on Vaccine Safety, 9–10 June 2005.Wkly Epidemiol Rec 2005, 80:242–247.
Hoke CH, Nisalak A, Sangawhipa N, et al.: Protection against Japanese encephalitis by inactivated vaccines. N Engl J Med 1988, 319:608–614.
Beasley DW, Lewthwaite P, Solomon T: Current use and development of vaccines for Japanese encephalitis. Expert Opin Biol Ther 2008, 8:95–106.
Andersen MM, Ronne T: Side-effects with Japanese encephalitis vaccine. Lancet 1991, 337:1044.
Ruff TA, Eisen D, Fuller A, et al.: Adverse reactions to Japanese encephalitis vaccine. Lancet 1991, 338:881–882.
Bista MB, Banerjee MK, Shin SH, et al.: Efficacy of single-dose SA 14-14-2 vaccine against Japanese encephalitis: a case control study. Lancet 2001, 358:791–795.
Ohrr H, Tandan JB, Sohn YM, et al.: Effect of single dose of SA 14-14-2 vaccine 1 year after immunisation in Nepalese children with Japanese encephalitis: a case-control study. Lancet 2005, 366:1375–1378.
Tandan JB, Ohrr H, Sohn YM, et al.: Single dose of SA 14-14-2 vaccine provides long-term protection against Japanese encephalitis: a case-control study in Nepalese children 5 years after immunization. Vaccine 2007, 25:5041–5045.
Kumar R, Tripathi P, Rizvi A: Effectiveness of one dose of SA 14-14-2 vaccine against Japanese encephalitis. N Engl J Med 2009, 360:1465–1466.
Gatchalian S, Yao Y, Zhou B, et al.: Comparison of the immunogenicity and safety of measles vaccine administered alone or with live, attenuated Japanese encephalitis SA 14-14-2 vaccine in Philippine infants. Vaccine 2008, 26:2234–2241.
Solomon T: Control of Japanese encephalitis—within our grasp? N Engl J Med 2006, 355:9:869–871.
• Kollaritsch H, Paulke-Korinek M, Dubischar-Kastner K: IC51 Japanese encephalitis vaccine. Expert Opin Biol Ther 2009, 9:7:921–931. This article provides an excellent overview of IC51 development history and pathway.
Eckels KH, Yu YX, Dubois DR, et al.: Japanese encephalitis virus live-attenuated vaccine, Chinese strain SA14-14-2; adaptation to primary canine kidney cell cultures and preparation of a vaccine for human use. Vaccine 1988, 6: 513–518.
Montagnon BJ. Polio and rabies vaccines produced in continuous cell lines: a reality for Vero cell line. Devel Biol Stand 1989, 70:27–47.
Srivastava AK, Putnak JR, Lee SH, et al. A purified inactivated Japanese encephalitis virus vaccine made in Vero cells. Vaccine 2001, 19:4557–4565.
Lyons A, Kanesa-thasan N, Kuschner RA, et al.: A Phase 2 study of a purified, inactivated virus vaccine to prevent Japanese encephalitis. Vaccine 2007, 25:17:3445–3453.
Tauber E, Kollaritsch H, Korinek M, et al.: Safety and immunogenicity of a Vero-cell-derived, inactivated Japanese encephalitis vaccine: a non-inferiority, phase III, randomised controlled trial. Lancet 2007, 370:1847–1853.
Tauber E, Kollaritsch H, von Sonnenburg F, et al.: Randomized, double-blind, placebo-controlled phase 3 trial of the safety and tolerability of IC51, an inactivated Japanese encephalitis vaccine. J Infect Dis 2008, 198:493–499.
Schuller E, Jilma B, Voicu V, et al.: Long-term immunogenicity of the new Vero cell-derived, inactivated Japanese encephalitis virus vaccine IC51 Six and 12 month results of a multicenter follow-up phase 3 study. Vaccine 2008, 26:4382–4386.
Schuller E, Klade CS, Wolfl G, et al.: Comparison of a single, high-dose vaccination regimen to the standard regimen for the investigational Japanese encephalitis vaccine, IC51: a randomized, observer-blind, controlled Phase 3 study. Vaccine 2009, 27:2188–2193.
Schuller E, Klade CS, Heinz FX, et al.: Effect of pre-existing anti-tick-borne encephalitis virus immunity on neutralising antibody response to the Vero cell-derived, inactivated Japanese encephalitis virus vaccine candidate IC51. Vaccine 2008, 26:6151–6156.
Kaltenbock A, Dubischar-Kastner K, Eder G, et al.: Safety and immunogenicity of concomitant vaccination with the cell-culture based Japanese Encephalitis vaccine IC51 and the hepatitis A vaccine HAVRIX1440 in healthy subjects: A single-blind, randomized, controlled Phase 3 study. Vaccine 2009, 27:4483–4489.
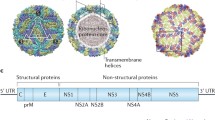
Chambers TJ, Nestorowicz A, Mason PW, Rice CM: Yellow fever/Japanese encephalitis chimeric viruses: construction and biological properties. J Virol 1999, 73:3095–3101.
Monath TP, Soike K, Levenbook I, et al.: Recombinant, chimaeric live, attenuated vaccine (ChimeriVax) incorporating the envelope genes of Japanese encephalitis (SA14-14-2) virus and the capsid and nonstructural genes of yellow fever (17D) virus is safe, immunogenic and protective in non-human primates. Vaccine 1999, 17:1869–1882.
Monath TP, McCarthy K, Bedford P, et al.: Clinical proof of principle for ChimeriVax: recombinant live, attenuated vaccines against flavivirus infections. Vaccine 2002, 20:1004–1018.
Monath TP, Guirakhoo F, Nichols R, et al. Chimeric live, attenuated vaccine against Japanese encephalitis (ChimeriVax-JE): phase 2 clinical trials for safety and immunogenicity, effect of vaccine dose and schedule, and memory response to challenge with inactivated Japanese encephalitis antigen. J Infect Dis 2003, 188:1213–1230.
Disclosure
One of the authors (SJT) was an associate investigator on a clinical trial evaluating the JE-PIV (IC51, Ixiaro) vaccine. This statement is made in the interest of full disclosure and not because the authors consider this to be a conflict of interest. No other potential conflict of interest relevant to this article was reported.
Disclaimer
The opinions or assertions contained herein are the private views of the author (SJT) and are not to be construed as reflecting the official views of the United States Army or the United States Department of Defense.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Halstead, S.B., Thomas, S.J. New Vaccines for Japanese Encephalitis. Curr Infect Dis Rep 12, 174–180 (2010). https://doi.org/10.1007/s11908-010-0098-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11908-010-0098-z