Abstract
Purpose of Review
Preeclampsia complicates 5–10% of all pregnancies and is a leading cause of maternal and perinatal mortality and morbidity. The placenta plays a pivotal role in determining pregnancy outcome by supplying the fetus with oxygen and nutrients and by synthesizing hormones. Placental function is highly dependent on energy supplied by mitochondria. It is well-known that preeclampsia is originated from placental dysfunction, although the etiology of it remains elusive.
Recent Findings
During the last three decades, substantial evidence suggests that mitochondrial abnormality is a major contributor to placental dysfunction. In addition, mitochondrial damage caused by circulating bioactive factors released from the placenta may cause endothelial dysfunction and subsequent elevation in maternal blood pressure.
Summary
In this review, we summarize the current knowledge of mitochondrial abnormality in the pathogenesis of preeclampsia and discuss therapeutic approaches targeting mitochondria for treatment of preeclampsia.


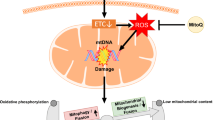
source of cellular reactive oxygen species (ROS). Leakage of electrons at complexes I and III from the ETC leads to partial reduction of oxygen to form superoxide (O2•−). O2•− is rapidly converted to hydrogen peroxide (H2O2) by superoxide dismutases (SODs), which is then reduced to water by glutathione peroxidases (GPXs) and peroxiredoxins (PRXs). The production of ROS at complexes I and III are increased in hypoxic conditions
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Roger AJ, Munoz-Gomez SA, Kamikawa R. The origin and diversification of mitochondria. Curr Biol. 2017;27(21):R1177–92. https://doi.org/10.1016/j.cub.2017.09.015.
Roca-Portoles A, Tait SWG. Mitochondrial quality control: from molecule to organelle. Cell Mol Life Sci. 2021;78(8):3853–66. https://doi.org/10.1007/s00018-021-03775-0.
Eisner V, Picard M, Hajnoczky G. Mitochondrial dynamics in adaptive and maladaptive cellular stress responses. Nat Cell Biol. 2018;20(7):755–65. https://doi.org/10.1038/s41556-018-0133-0.
Javadov S, Kozlov AV, Camara AKS. Mitochondria in health and diseases. Cells. 2020;9(5). https://doi.org/10.3390/cells9051177
Burton GJ, Fowden AL, Thornburg KL. Placental origins of chronic disease. Physiol Rev. 2016;96(4):1509–65. https://doi.org/10.1152/physrev.00029.2015.
• Michelsen TM, Holme AM, Holm MB, Roland MC, Haugen G, Powell TL, et al. Uteroplacental Glucose Uptake and Fetal Glucose Consumption: A Quantitative Study in Human Pregnancies. J Clin Endocrinol Metab. 2019;104(3):873-82. https://doi.org/10.1210/jc.2018-01154. This study described glucose consumption in the human placenta.
• Carter AM. Placental oxygen consumption. Part I: in vivo studies - a review. Placenta. 2000;21 Suppl A:S31–7. https://doi.org/10.1053/plac.1999.0513. This study described oxygen consumption in the placenta.
Vaughan OR, Fowden AL. Placental metabolism: substrate requirements and the response to stress. Reprod Domest Anim. 2016;51(Suppl 2):25–35. https://doi.org/10.1111/rda.12797.
Gestational Hypertension and Preeclampsia. ACOG Practice Bulletin, Number 222. Obstet Gynecol. 2020;135(6):e237–60. https://doi.org/10.1097/AOG.0000000000003891.
Rana S, Lemoine E, Granger JP, Karumanchi SA. Preeclampsia: pathophysiology, challenges, and perspectives. Circ Res. 2019;124(7):1094–112. https://doi.org/10.1161/CIRCRESAHA.118.313276.
Melchiorre K, Thilaganathan B, Giorgione V, Ridder A, Memmo A, Khalil A. Hypertensive disorders of pregnancy and future cardiovascular health. Front Cardiovasc Med. 2020;7:59. https://doi.org/10.3389/fcvm.2020.00059.
•• Redman CW, Sargent IL. Latest advances in understanding preeclampsia. Science 2005;308(5728):1592–1594. https://doi.org/10.1126/science.1111726. The two-stage model of preeclampsia was discussed in this review paper.
•• Torbergsen T, Oian P, Mathiesen E, Borud O. Pre-eclampsia - a mitochondrial disease? Acta Obstet Gynecol Scand. 1989;68(2):145–148. https://doi.org/10.3109/00016348909009902. This observational study first described a high incidence of preeclampsia in a family with mitochondrial disorder.
Marin R, Chiarello DI, Abad C, Rojas D, Toledo F, Sobrevia L. Oxidative stress and mitochondrial dysfunction in early-onset and late-onset preeclampsia. Biochim Biophys Acta Mol Basis Dis. 2020;1866(12): 165961. https://doi.org/10.1016/j.bbadis.2020.165961.
Taylor CT. Mitochondria and cellular oxygen sensing in the HIF pathway. Biochem J. 2008;409(1):19–26. https://doi.org/10.1042/BJ20071249.
Ma LN, Huang XB, Muyayalo KP, Mor G, Liao AH. Lactic Acid: A Novel Signaling Molecule in Early Pregnancy? Front Immunol. 2020;11:279. https://doi.org/10.3389/fimmu.2020.00279.
•• Jauniaux E, Watson AL, Hempstock J, Bao YP, Skepper JN, Burton GJ. Onset of maternal arterial blood flow and placental oxidative stress. A possible factor in human early pregnancy failure. Am J Pathol. 2000;157(6):2111–22. https://doi.org/10.1016/S0002-9440(10)64849-3. The study examined the placental oxygen levels before and after the establishment of the uteroplacental circulation.
Schneider H. Placental oxygen consumption. Part II: in vitro studies - a review. Placenta. 2000;21 Suppl A:S38–44. https://doi.org/10.1053/plac.1999.0512
Yu J, Guo X, Chen R, Feng L. Downregulation of Mitofusin 2 in Placenta Is Related to Preeclampsia. Biomed Res Int. 2016;2016:6323086. https://doi.org/10.1155/2016/6323086.
Zhou X, Han TL, Chen H, Baker PN, Qi H, Zhang H. Impaired mitochondrial fusion, autophagy, biogenesis and dysregulated lipid metabolism is associated with preeclampsia. Exp Cell Res. 2017;359(1):195–204. https://doi.org/10.1016/j.yexcr.2017.07.029.
Sato E, Tsunokuni Y, Kaneko M, Saigusa D, Saito R, Shimma S, et al. Metabolomics of a mouse model of preeclampsia induced by overexpressing soluble fms-like tyrosine kinase 1. Biochem Biophys Res Commun. 2020;527(4):1064–71. https://doi.org/10.1016/j.bbrc.2020.04.079.
•• Soleymanlou N, Jurisica I, Nevo O, Ietta F, Zhang X, Zamudio S, et al. Molecular evidence of placental hypoxia in preeclampsia J Clin Endocrinol Metab. 2005;90(7):4299–4308. https://doi.org/10.1210/jc.2005-0078. This study demonstrated that the preeclamptic placenta exhibited hypoxic phenotype.
•• Semenza GL, Roth PH, Fang HM, Wang GL. Transcriptional regulation of genes encoding glycolytic enzymes by hypoxia-inducible factor 1. J Biol Chem. 1994;269(3):8 23757 23763. This study demonstrated the reprogramming cell bioengeretics in hypoxia.
Sferruzzi-Perri AN, Higgins JS, Vaughan OR, Murray AJ, Fowden AL. Placental mitochondria adapt developmentally and in response to hypoxia to support fetal growth. Proc Natl Acad Sci U S A. 2019;116(5):1621–6. https://doi.org/10.1073/pnas.1816056116.
•• Vangrieken P, Al-Nasiry S, Bast A, Leermakers PA, Tulen CBM, Schiffers PMH et al. Placental Mitochondrial Abnormalities in Preeclampsia. Reprod Sci. 2021. https://doi.org/10.1007/s43032-021-00464-y. This study thoroughly examined various aspects of mitochondrial dysfunction in the preeclamptic placenta.
Shi Z, Long W, Zhao C, Guo X, Shen R, Ding H. Comparative proteomics analysis suggests that placental mitochondria are involved in the development of pre-eclampsia. PLoS ONE. 2013;8(5): e64351. https://doi.org/10.1371/journal.pone.0064351.
Xu Z, Jin X, Cai W, Zhou M, Shao P, Yang Z, et al. Proteomics analysis reveals abnormal electron transport and excessive oxidative stress cause mitochondrial dysfunction in placental tissues of early-onset preeclampsia. Proteomics Clin Appl. 2018;12(5): e1700165. https://doi.org/10.1002/prca.201700165.
Matsubara S, Minakami H, Sato I, Saito T. Decrease in cytochrome c oxidase activity detected cytochemically in the placental trophoblast of patients with pre-eclampsia. Placenta. 1997;18(4):255–9. https://doi.org/10.1016/s0143-4004(97)80059-8.
Muralimanoharan S, Maloyan A, Mele J, Guo C, Myatt LG, Myatt L. MIR-210 modulates mitochondrial respiration in placenta with preeclampsia. Placenta. 2012;33(10):816–23. https://doi.org/10.1016/j.placenta.2012.07.002.
Beyramzadeh M, Dikmen ZG, Erturk NK, Tuncer ZS, Akbiyik F. Placental respiratory chain complex activities in high risk pregnancies. J Matern Fetal Neonatal Med. 2017;30(24):2911–7. https://doi.org/10.1080/14767058.2016.1268594.
Covarrubias AE, Lecarpentier E, Lo A, Salahuddin S, Gray KJ, Karumanchi SA, et al. AP39, a modulator of mitochondrial bioenergetics, reduces antiangiogenic response and oxidative stress in hypoxia-exposed trophoblasts: relevance for preeclampsia pathogenesis. Am J Pathol. 2019;189(1):104–14. https://doi.org/10.1016/j.ajpath.2018.09.007.
•• Holland OJ, Cuffe JSM, Dekker Nitert M, Callaway L, Kwan Cheung KA, Radenkovic F et al. Placental mitochondrial adaptations in preeclampsia associated with progression to term delivery. Cell Death Dis. 2018;9(12):1150. https://doi.org/10.1038/s41419-018-1190-9 . This study examined and compared mitochondrial function/dysfunction in the placenta of normal pregnancy, early-onst and late-onset preeclampsia.
Yung HW, Colleoni F, Dommett E, Cindrova-Davies T, Kingdom J, Murray AJ, et al. Noncanonical mitochondrial unfolded protein response impairs placental oxidative phosphorylation in early-onset preeclampsia. Proc Natl Acad Sci U S A. 2019;116(36):18109–18. https://doi.org/10.1073/pnas.1907548116.
Holland OJ, Hickey AJR, Alvsaker A, Moran S, Hedges C, Chamley LW, et al. Changes in mitochondrial respiration in the human placenta over gestation. Placenta. 2017;57:102–12. https://doi.org/10.1016/j.placenta.2017.06.011.
Bartha JL, Visiedo F, Fernandez-Deudero A, Bugatto F, Perdomo G. Decreased mitochondrial fatty acid oxidation in placentas from women with preeclampsia. Placenta. 2012;33(2):132–4. https://doi.org/10.1016/j.placenta.2011.11.027.
Thomas MM, Haghiac M, Grozav C, Minium J, Calabuig-Navarro V, O’Tierney-Ginn P. Oxidative stress impairs fatty acid oxidation and mitochondrial function in the term placenta. Reprod Sci. 2019;26(7):972–8. https://doi.org/10.1177/1933719118802054.
• Illsley NP, Caniggia I, Zamudio S. Placental metabolic reprogramming: do changes in the mix of energy-generating substrates modulate fetal growth? Int J Dev Biol. 2010;54(2-3):409-19. https://doi.org/10.1387/ijdb.082798ni. The review discussed metabolic reprogramming in the placenta under hypoxic conditions.
Colleoni F, Padmanabhan N, Yung HW, Watson ED, Cetin I, Tissot van Patot MC, et al. Suppression of mitochondrial electron transport chain function in the hypoxic human placenta: a role for miRNA-210 and protein synthesis inhibition. PLoS One. 2013;8(1):e55194. https://doi.org/10.1371/journal.pone.0055194.
•• Vangrieken P, Al-Nasiry S, Bast A, Leermakers PA, Tulen CBM, Janssen GMJ, et al. Hypoxia-induced mitochondrial abnormalities in cells of the placenta. PLoS One. 2021;16(1):e0245155. https://doi.org/10.1371/journal.pone.0245155. This study demonstrated that many features of mitochondrial dusfunction in the preeclamptic placenta could be simulated by hypxoic exposure.
• Vaka VR, McMaster KM, Cunningham Jr MW, Ibrahim T, Hazlewood R, Usry N, et al. Role of Mitochondrial Dysfunction and Reactive Oxygen Species in Mediating Hypertension in the Reduced Uterine Perfusion Pressure Rat Model of Preeclampsia. Hypertension. 2018;72(3):703-711. https://doi.org/10.1161/HYPERTENSIONAHA.118.11290. This study demonstrated that mitochondrial dysfunction was recapitulate in an animal model of preeclampsia.
Chen G, Lin Y, Chen L, Zeng F, Zhang L, Huang Y, et al. Role of DRAM1 in mitophagy contributes to preeclampsia regulation in mice. Mol Med Rep. 2020;22(3):1847–58. https://doi.org/10.3892/mmr.2020.11269.
Ganguly E, Kirschenman R, Spaans F, Holody CD, Phillips TEJ, Case CP, et al. Nanoparticle-encapsulated antioxidant improves placental mitochondrial function in a sexually dimorphic manner in a rat model of prenatal hypoxia. FASEB J. 2021;35(2): e21338. https://doi.org/10.1096/fj.202002193R.
Bloxam DL, Bullen BE, Walters BN, Lao TT. Placental glycolysis and energy metabolism in preeclampsia. Am J Obstet Gynecol. 1987;157(1):97–101. https://doi.org/10.1016/s0002-9378(87)80354-x.
Murphy MP. How mitochondria produce reactive oxygen species. Biochem J. 2009;417(1):1–13. https://doi.org/10.1042/BJ20081386.
Garcia-Santamarina S, Boronat S, Hidalgo E. Reversible cysteine oxidation in hydrogen peroxide sensing and signal transduction. Biochemistry. 2014;53(16):2560–80. https://doi.org/10.1021/bi401700f.
Lee P, Chandel NS, Simon MC. Cellular adaptation to hypoxia through hypoxia inducible factors and beyond. Nat Rev Mol Cell Biol. 2020;21(5):268–83. https://doi.org/10.1038/s41580-020-0227-y.
Zorov DB, Juhaszova M, Sollott SJ. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol Rev. 2014;94(3):909–50. https://doi.org/10.1152/physrev.00026.2013.
Chiarello DI, Abad C, Rojas D, Toledo F, Vazquez CM, Mate A, et al. Oxidative stress: normal pregnancy versus preeclampsia. Biochim Biophys Acta Mol Basis Dis. 2020;1866(2): 165354. https://doi.org/10.1016/j.bbadis.2018.12.005.
• Wang Y, Walsh SW. Placental mitochondria as a source of oxidative stress in pre-eclampsia. Placenta. 1998;19(8):581-6. https://doi.org/10.1016/s0143-4004(98)90018-2. This study provided first evidence of elevated mitochondrial reactive oxygen species in the preeclamptic placenta.
Aljunaidy MM, Morton JS, Kirschenman R, Phillips T, Case CP, Cooke CM, et al. Maternal treatment with a placental-targeted antioxidant (MitoQ) impacts offspring cardiovascular function in a rat model of prenatal hypoxia. Pharmacol Res. 2018;134:332–42. https://doi.org/10.1016/j.phrs.2018.05.006.
Zamudio S, Kovalenko O, Vanderlelie J, Illsley NP, Heller D, Belliappa S, et al. Chronic hypoxia in vivo reduces placental oxidative stress. Placenta. 2007;28(8–9):846–53. https://doi.org/10.1016/j.placenta.2006.11.010.
Kohan-Ghadr HR, Kilburn BA, Kadam L, Johnson E, Kolb BL, Rodriguez-Kovacs J, et al. Rosiglitazone augments antioxidant response in the human trophoblast and prevents apoptosisdagger. Biol Reprod. 2019;100(2):479–94. https://doi.org/10.1093/biolre/ioy186.
Rueda-Clausen CF, Stanley JL, Thambiraj DF, Poudel R, Davidge ST, Baker PN. Effect of prenatal hypoxia in transgenic mouse models of preeclampsia and fetal growth restriction. Reprod Sci. 2014;21(4):492–502. https://doi.org/10.1177/1933719113503401.
•• Yang Y, Xu P, Zhu F, Liao J, Wu Y, Hu M, et al. The potent antioxidant mitoq protects against preeclampsia during late gestation but increases the risk of preeclampsia when administered in early pregnancy. Antioxid Redox Signal. 2021;34(2):118-36. https://doi.org/10.1089/ars.2019.7891. This study examined the time frame in starting anoxidant treatment in an animal model of preeclampsia and demonstrated the effectiveness of mitochondria-targeted compound mitoQ in alleviating preeclampsia-like symptoms.
Ding D, Scott NM, Thompson EE, Chaiworapongsa T, Torres R, Billstrand C, et al. Increased protein-coding mutations in the mitochondrial genome of African American women with preeclampsia. Reprod Sci. 2012;19(12):1343–51. https://doi.org/10.1177/1933719112450337.
Cline SD. Mitochondrial DNA damage and its consequences for mitochondrial gene expression. Biochim Biophys Acta. 2012;1819(9–10):979–91. https://doi.org/10.1016/j.bbagrm.2012.06.002.
Kowaltowski AJ, Castilho RF, Vercesi AE. Mitochondrial permeability transition and oxidative stress. FEBS Lett. 2001;495(1–2):12–5. https://doi.org/10.1016/s0014-5793(01)02316-x.
Tomas SZ, Prusac IK, Roje D, Tadin I. Trophoblast apoptosis in placentas from pregnancies complicated by preeclampsia. Gynecol Obstet Invest. 2011;71(4):250–5. https://doi.org/10.1159/000320289.
Murata M, Fukushima K, Takao T, Seki H, Takeda S, Wake N. Oxidative stress produced by xanthine oxidase induces apoptosis in human extravillous trophoblast cells. J Reprod Dev. 2013;59(1):7–13. https://doi.org/10.1262/jrd.2012-053.
Zhou X, Zhang GY, Wang J, Lu SL, Cao J, Sun LZ. A novel bridge between oxidative stress and immunity: the interaction between hydrogen peroxide and human leukocyte antigen G in placental trophoblasts during preeclampsia. Am J Obstet Gynecol. 2012;206(5):447 e7–16. https://doi.org/10.1016/j.ajog.2012.03.013
Walker OS, Ragos R, Wong MK, Adam M, Cheung A, Raha S. Reactive oxygen species from mitochondria impacts trophoblast fusion and the production of endocrine hormones by syncytiotrophoblasts. PLoS ONE. 2020;15(2): e0229332. https://doi.org/10.1371/journal.pone.0229332.
Ushida T, Kotani T, Tsuda H, Imai K, Nakano T, Hirako S, et al. Molecular hydrogen ameliorates several characteristics of preeclampsia in the Reduced Uterine Perfusion Pressure (RUPP) rat model. Free Radic Biol Med. 2016;101:524–33. https://doi.org/10.1016/j.freeradbiomed.2016.10.491.
Zemirli N, Morel E, Molino D. Mitochondrial dynamics in basal and stressful conditions. Int J Mol Sci. 2018;19(2). https://doi.org/10.3390/ijms19020564
Youle RJ, van der Bliek AM. Mitochondrial fission, fusion, and stress. Science. 2012;337(6098):1062–5. https://doi.org/10.1126/science.1219855.
Mishra P, Chan DC. Metabolic regulation of mitochondrial dynamics. J Cell Biol. 2016;212(4):379–87. https://doi.org/10.1083/jcb.201511036.
Ausman J, Abbade J, Ermini L, Farrell A, Tagliaferro A, Post M, et al. Ceramide-induced BOK promotes mitochondrial fission in preeclampsia. Cell Death Dis. 2018;9(3):298. https://doi.org/10.1038/s41419-018-0360-0.
•• Vishnyakova PA, Volodina MA, Tarasova NV, Marey MV, Tsvirkun DV, Vavina OV, et al. Mitochondrial role in adaptive response to stress conditions in preeclampsia. Sci Rep. 2016;6:32410. https://doi.org/10.1038/srep32410. This study also examined various aspects of mitochondrial function/dysfunction in the placenta of normal pregnancy, early-onst and late-onset preeclampsia and demonstrated that mitochondria underwent adaptative changes in preeclampsia.
Liu Y, Beyer A, Aebersold R. On the dependency of cellular protein levels on mRNA abundance. Cell. 2016;165(3):535–50. https://doi.org/10.1016/j.cell.2016.03.014.
Kubli DA, Ycaza JE, Gustafsson AB. BNIP3 mediates mitochondrial dysfunction and cell death through Bax and Bak. Biochem J. 2007;405(3):407–15. https://doi.org/10.1042/BJ20070319.
Zhang J, Ney PA. Role of BNIP3 and NIX in cell death, autophagy, and mitophagy. Cell Death Differ. 2009;16(7):939–46. https://doi.org/10.1038/cdd.2009.16.
Liu L, Feng D, Chen G, Chen M, Zheng Q, Song P, et al. Mitochondrial outer-membrane protein FUNDC1 mediates hypoxia-induced mitophagy in mammalian cells. Nat Cell Biol. 2012;14(2):177–85. https://doi.org/10.1038/ncb2422.
Wenz T. Regulation of mitochondrial biogenesis and PGC-1alpha under cellular stress. Mitochondrion. 2013;13(2):134–42. https://doi.org/10.1016/j.mito.2013.01.006.
Canto C, Auwerx J. PGC-1alpha, SIRT1 and AMPK, an energy sensing network that controls energy expenditure. Curr Opin Lipidol. 2009;20(2):98–105. https://doi.org/10.1097/MOL.0b013e328328d0a4.
Scarpulla RC. Transcriptional paradigms in mammalian mitochondrial biogenesis and function. Physiol Rev. 2008;88(2):611–38. https://doi.org/10.1152/physrev.00025.2007.
Filograna R, Mennuni M, Alsina D, Larsson NG. Mitochondrial DNA copy number in human disease: the more the better? FEBS Lett. 2021;595(8):976–1002. https://doi.org/10.1002/1873-3468.14021.
Knyazev EN, Zakharova GS, Astakhova LA, Tsypina IM, Tonevitsky AG, Sukhikh GT. Metabolic reprogramming of trophoblast cells in response to hypoxia. Bull Exp Biol Med. 2019;166(3):321–5. https://doi.org/10.1007/s10517-019-04342-1.
Kumar S, Gordon GH, Abbott DH, Mishra JS. Androgens in maternal vascular and placental function: implications for preeclampsia pathogenesis. Reproduction. 2018;156(5):R155–67. https://doi.org/10.1530/REP-18-0278.
Mishra JS, Blesson CS, Kumar S. Testosterone decreases placental mitochondrial content and cellular bioenergetics. Biology (Basel). 2020;9(7). https://doi.org/10.3390/biology9070176.
Mando C, De Palma C, Stampalija T, Anelli GM, Figus M, Novielli C, et al. Placental mitochondrial content and function in intrauterine growth restriction and preeclampsia. Am J Physiol Endocrinol Metab. 2014;306(4):E404–13. https://doi.org/10.1152/ajpendo.00426.2013.
Bock FJ, Tait SWG. Mitochondria as multifaceted regulators of cell death. Nat Rev Mol Cell Biol. 2020;21(2):85–100. https://doi.org/10.1038/s41580-019-0173-8.
Kroemer G, Galluzzi L, Brenner C. Mitochondrial membrane permeabilization in cell death. Physiol Rev. 2007;87(1):99–163. https://doi.org/10.1152/physrev.00013.2006.
Kinnally KW, Peixoto PM, Ryu SY, Dejean LM. Is mPTP the gatekeeper for necrosis, apoptosis, or both? Biochim Biophys Acta. 2011;1813(4):616–22. https://doi.org/10.1016/j.bbamcr.2010.09.013.
Ma L, Zhang Z, Dong K, Ma Y. TWIST1 Alleviates hypoxia-induced damage of trophoblast cells by inhibiting mitochondrial apoptosis pathway. Exp Cell Res. 2019;385(2): 111687. https://doi.org/10.1016/j.yexcr.2019.111687.
Longtine MS, Chen B, Odibo AO, Zhong Y, Nelson DM. Villous trophoblast apoptosis is elevated and restricted to cytotrophoblasts in pregnancies complicated by preeclampsia, IUGR, or preeclampsia with IUGR. Placenta. 2012;33(5):352–9. https://doi.org/10.1016/j.placenta.2012.01.017.
Ishihara N, Matsuo H, Murakoshi H, Laoag-Fernandez JB, Samoto T, Maruo T. Increased apoptosis in the syncytiotrophoblast in human term placentas complicated by either preeclampsia or intrauterine growth retardation. Am J Obstet Gynecol. 2002;186(1):158–66. https://doi.org/10.1067/mob.2002.119176.
Heazell AE, Buttle HR, Baker PN, Crocker IP. Altered expression of regulators of caspase activity within trophoblast of normal pregnancies and pregnancies complicated by preeclampsia. Reprod Sci. 2008;15(10):1034–43. https://doi.org/10.1177/1933719108322438.
Park JK, Kang MY, Kim YH, Jo HC, Shin JK, Choi WJ, et al. PKC delta in preeclamptic placentas promotes Bax dissociation from 14–3-3 zeta through 14–3-3 zeta phosphorylation. Placenta. 2008;29(7):584–92. https://doi.org/10.1016/j.placenta.2008.03.007.
Hung TH, Chen SF, Liou JD, Hsu JJ, Li MJ, Yeh YL, et al. Bax, Bak and mitochondrial oxidants are involved in hypoxia-reoxygenation-induced apoptosis in human placenta. Placenta. 2008;29(7):565–83. https://doi.org/10.1016/j.placenta.2008.03.005.
Hu R, Zhou S, Li X. Altered Bcl-2 and Bax expression is associated with cultured first trimester human cytotrophoblasts apoptosis induced by hypoxia. Life Sci. 2006;79(4):351–5. https://doi.org/10.1016/j.lfs.2006.01.011.
Gilbert JS, Ryan MJ, LaMarca BB, Sedeek M, Murphy SR, Granger JP. Pathophysiology of hypertension during preeclampsia: linking placental ischemia with endothelial dysfunction. Am J Physiol Heart Circ Physiol. 2008;294(2):H541–50. https://doi.org/10.1152/ajpheart.01113.2007.
Qu H, Khalil RA. Vascular mechanisms and molecular targets in hypertensive pregnancy and preeclampsia. Am J Physiol Heart Circ Physiol. 2020;319(3):H661–81. https://doi.org/10.1152/ajpheart.00202.2020.
•• Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest. 2003;111(5):649-58. https://doi.org/10.1172/JCI17189. This study provided evidence that sFlt-1 released from the preeclamptic placenta caused endothelial dysfunction and clinical symptoms of preeclampsia.
Shibata E, Rajakumar A, Powers RW, Larkin RW, Gilmour C, Bodnar LM, et al. Soluble fms-like tyrosine kinase 1 is increased in preeclampsia but not in normotensive pregnancies with small-for-gestational-age neonates: relationship to circulating placental growth factor. J Clin Endocrinol Metab. 2005;90(8):4895–903. https://doi.org/10.1210/jc.2004-1955.
Levine RJ, Maynard SE, Qian C, Lim KH, England LJ, Yu KF, et al. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med. 2004;350(7):672–83. https://doi.org/10.1056/NEJMoa031884.
Holme AM, Roland MC, Henriksen T, Michelsen TM. In vivo uteroplacental release of placental growth factor and soluble Fms-like tyrosine kinase-1 in normal and preeclamptic pregnancies. Am J Obstet Gynecol. 2016;215(6):782 e1- e9. https://doi.org/10.1016/j.ajog.2016.07.056.
Walentowicz-Sadlecka M, Domaracki P, Sadlecki P, Siodmiak J, Grabiec M, Walentowicz P, et al. Assessment of the SFlt-1 and sFlt-1/25(OH)D ratio as a diagnostic tool in gestational hypertension (GH), preeclampsia (PE), and gestational diabetes mellitus (GDM). Dis Markers. 2019;2019:5870239. https://doi.org/10.1155/2019/5870239.
Gilbert JS, Babcock SA, Granger JP. Hypertension produced by reduced uterine perfusion in pregnant rats is associated with increased soluble fms-like tyrosine kinase-1 expression. Hypertension. 2007;50(6):1142–7. https://doi.org/10.1161/HYPERTENSIONAHA.107.096594.
Ahmad S, Ahmed A. Elevated placental soluble vascular endothelial growth factor receptor-1 inhibits angiogenesis in preeclampsia. Circ Res. 2004;95(9):884–91. https://doi.org/10.1161/01.RES.0000147365.86159.f5.
Nevo O, Soleymanlou N, Wu Y, Xu J, Kingdom J, Many A, et al. Increased expression of sFlt-1 in in vivo and in vitro models of human placental hypoxia is mediated by HIF-1. Am J Physiol Regul Integr Comp Physiol. 2006;291(4):R1085–93. https://doi.org/10.1152/ajpregu.00794.2005.
Gerber HP, Condorelli F, Park J, Ferrara N. Differential transcriptional regulation of the two vascular endothelial growth factor receptor genes. Flt-1, but not Flk-1/KDR, is up-regulated by hypoxia. J Biol Chem. 1997;272(38):23659–67. https://doi.org/10.1074/jbc.272.38.23659.
Bergmann A, Ahmad S, Cudmore M, Gruber AD, Wittschen P, Lindenmaier W, et al. Reduction of circulating soluble Flt-1 alleviates preeclampsia-like symptoms in a mouse model. J Cell Mol Med. 2010;14(6B):1857–67. https://doi.org/10.1111/j.1582-4934.2009.00820.x.
Chang X, Yao J, He Q, Liu M, Duan T, Wang K. Exosomes from women with preeclampsia induced vascular dysfunction by delivering sFlt (Soluble Fms-Like Tyrosine Kinase)-1 and sEng (Soluble Endoglin) to endothelial cells. Hypertension. 2018;72(6):1381–90. https://doi.org/10.1161/HYPERTENSIONAHA.118.11706.
Sanchez-Aranguren LC, Espinosa-Gonzalez CT, Gonzalez-Ortiz LM, Sanabria-Barrera SM, Riano-Medina CE, Nunez AF, et al. Soluble Fms-Like Tyrosine Kinase-1 Alters Cellular Metabolism and Mitochondrial Bioenergetics in Preeclampsia. Front Physiol. 2018;9:83. https://doi.org/10.3389/fphys.2018.00083.
Bridges JP, Gilbert JS, Colson D, Gilbert SA, Dukes MP, Ryan MJ, et al. Oxidative stress contributes to soluble fms-like tyrosine kinase-1 induced vascular dysfunction in pregnant rats. Am J Hypertens. 2009;22(5):564–8. https://doi.org/10.1038/ajh.2009.24.
McArthur K, Whitehead LW, Heddleston JM, Li L, Padman BS, Oorschot V, et al. BAK/BAX macropores facilitate mitochondrial herniation and mtDNA efflux during apoptosis. Science. 2018;359(6378). doi:https://doi.org/10.1126/science.aao6047.
Riley JS, Tait SW. Mitochondrial DNA in inflammation and immunity. EMBO Rep. 2020;21(4): e49799. https://doi.org/10.15252/embr.201949799.
Zhang Q, Raoof M, Chen Y, Sumi Y, Sursal T, Junger W, et al. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 2010;464(7285):104–7. https://doi.org/10.1038/nature08780.
Shimada K, Crother TR, Karlin J, Dagvadorj J, Chiba N, Chen S, et al. Oxidized mitochondrial DNA activates the NLRP3 inflammasome during apoptosis. Immunity. 2012;36(3):401–14. https://doi.org/10.1016/j.immuni.2012.01.009.
Bai B, Yang Y, Wang Q, Li M, Tian C, Liu Y, et al. NLRP3 inflammasome in endothelial dysfunction. Cell Death Dis. 2020;11(9):776. https://doi.org/10.1038/s41419-020-02985-x.
Zhou R, Yazdi AS, Menu P, Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011;469(7329):221–5. https://doi.org/10.1038/nature09663.
Williamson RD, McCarthy FP, Khashan AS, Totorika A, Kenny LC, McCarthy C. Exploring the role of mitochondrial dysfunction in the pathophysiology of pre-eclampsia. Pregnancy Hypertens. 2018;13:248–53. https://doi.org/10.1016/j.preghy.2018.06.012.
Marschalek J, Wohlrab P, Ott J, Wojta J, Speidl W, Klein KU, et al. Maternal serum mitochondrial DNA (mtDNA) levels are elevated in preeclampsia - a matched case-control study. Pregnancy Hypertens. 2018;14:195–9. https://doi.org/10.1016/j.preghy.2018.10.003.
Orozco AF, Jorgez CJ, Ramos-Perez WD, Popek EJ, Yu X, Kozinetz CA, et al. Placental release of distinct DNA-associated micro-particles into maternal circulation: reflective of gestation time and preeclampsia. Placenta. 2009;30(10):891–7. https://doi.org/10.1016/j.placenta.2009.06.012.
Tong M, Johansson C, Xiao F, Stone PR, James JL, Chen Q, et al. Antiphospholipid antibodies increase the levels of mitochondrial DNA in placental extracellular vesicles: Alarmin-g for preeclampsia. Sci Rep. 2017;7(1):16556. https://doi.org/10.1038/s41598-017-16448-5.
Williamson RD, McCarthy FP, Kenny LC, McCarthy CM. Activation of a TLR9 mediated innate immune response in preeclampsia. Sci Rep. 2019;9(1):5920. https://doi.org/10.1038/s41598-019-42551-w.
• Goulopoulou S, Wenceslau CF, McCarthy CG, Matsumoto T, Webb RC. Exposure to stimulatory CpG oligonucleotides during gestation induces maternal hypertension and excess vasoconstriction in pregnant rats. Am J Physiol Heart Circ Physiol. 2016;310(8):H1015-25. https://doi.org/10.1152/ajpheart.00834.2015. The findings from this study implied that mtDNA released from the preeclamptic placenta could contribute to the development of preeclampsia.
Wallukat G, Homuth V, Fischer T, Lindschau C, Horstkamp B, Jupner A, et al. Patients with preeclampsia develop agonistic autoantibodies against the angiotensin AT1 receptor. J Clin Invest. 1999;103(7):945–52. https://doi.org/10.1172/JCI4106.
Xia Y, Wen H, Bobst S, Day MC, Kellems RE. Maternal autoantibodies from preeclamptic patients activate angiotensin receptors on human trophoblast cells. J Soc Gynecol Investig. 2003;10(2):82–93. https://doi.org/10.1016/s1071-5576(02)00259-9.
Xia Y, Kellems RE. Angiotensin receptor agonistic autoantibodies and hypertension: preeclampsia and beyond. Circ Res. 2013;113(1):78–87. https://doi.org/10.1161/CIRCRESAHA.113.300752.
• Zhou CC, Zhang Y, Irani RA, Zhang H, Mi T, Popek EJ, et al. Angiotensin receptor agonistic autoantibodies induce pre-eclampsia in pregnant mice. Nat Med. 2008;14(8):855-62. https://doi.org/10.1038/nm.1856. This study described that AT1-AA could induce preeclmapsia-like symptoms in pregnant mice.
LaMarca B, Wallukat G, Llinas M, Herse F, Dechend R, Granger JP. Autoantibodies to the angiotensin type I receptor in response to placental ischemia and tumor necrosis factor alpha in pregnant rats. Hypertension. 2008;52(6):1168–72. https://doi.org/10.1161/HYPERTENSIONAHA.108.120576.
Siddiqui AH, Irani RA, Zhang W, Wang W, Blackwell SC, Kellems RE, et al. Angiotensin receptor agonistic autoantibody-mediated soluble fms-like tyrosine kinase-1 induction contributes to impaired adrenal vasculature and decreased aldosterone production in preeclampsia. Hypertension. 2013;61(2):472–9. https://doi.org/10.1161/HYPERTENSIONAHA.111.00157.
McCarthy C, Kenny LC. Therapeutically targeting mitochondrial redox signalling alleviates endothelial dysfunction in preeclampsia. Sci Rep. 2016;6:32683. https://doi.org/10.1038/srep32683.
Deer E, Vaka VR, McMaster KM, Wallace K, Cornelius DC, Amaral LM, et al. Vascular endothelial mitochondrial oxidative stress in response to preeclampsia: a role for angiotension II type 1 autoantibodies. Am J Obstet Gynecol MFM. 2021;3(1): 100275. https://doi.org/10.1016/j.ajogmf.2020.100275.
Vaka VR, Cunningham MW, Deer E, Franks M, Ibrahim T, Amaral LM, et al. Blockade of endogenous angiotensin II type I receptor agonistic autoantibody activity improves mitochondrial reactive oxygen species and hypertension in a rat model of preeclampsia. Am J Physiol Regul Integr Comp Physiol. 2020;318(2):R256–62. https://doi.org/10.1152/ajpregu.00179.2019.
Bentinger M, Brismar K, Dallner G. The antioxidant role of coenzyme Q. Mitochondrion. 2007;7(Suppl):S41-50. https://doi.org/10.1016/j.mito.2007.02.006.
Xu X, Pan JR, Zhang YZ. CoQ10 alleviate preeclampsia symptoms by enhancing the function of mitochondria in the placenta of pregnant rats with preeclampsia. Hypertens Pregnancy. 2019;38(4):217–22. https://doi.org/10.1080/10641955.2019.1649420.
Teran E, Hernandez I, Nieto B, Tavara R, Ocampo JE, Calle A. Coenzyme Q10 supplementation during pregnancy reduces the risk of pre-eclampsia. Int J Gynaecol Obstet. 2009;105(1):43–5. https://doi.org/10.1016/j.ijgo.2008.11.033.
•• Murphy MP, Smith RA. Targeting antioxidants to mitochondria by conjugation to lipophilic cations. Annu Rev Pharmacol Toxicol. 2007;47:629-56. https://doi.org/10.1146/annurev.pharmtox.47.120505.105110. The review described the design of mitochondria-targeted compounds.
Phillips TJ, Scott H, Menassa DA, Bignell AL, Sood A, Morton JS, et al. Treating the placenta to prevent adverse effects of gestational hypoxia on fetal brain development. Sci Rep. 2017;7(1):9079. https://doi.org/10.1038/s41598-017-06300-1.
Ganguly E, Aljunaidy MM, Kirschenman R, Spaans F, Morton JS, Phillips TEJ, et al. Sex-Specific Effects of Nanoparticle-Encapsulated MitoQ (nMitoQ) Delivery to the placenta in a rat model of fetal hypoxia. Front Physiol. 2019;10:562. https://doi.org/10.3389/fphys.2019.00562.
Labunskyy VM, Hatfield DL, Gladyshev VN. Selenoproteins: molecular pathways and physiological roles. Physiol Rev. 2014;94(3):739–77. https://doi.org/10.1152/physrev.00039.2013.
Garcia-Garcia FJ, Monistrol-Mula A, Cardellach F, Garrabou G. Nutrition, Bioenergetics, and Metabolic Syndrome. Nutrients. 2020;12(9). https://doi.org/10.3390/nu12092785.
Khera A, Vanderlelie JJ, Holland O, Perkins AV. Overexpression of endogenous anti-oxidants with selenium supplementation protects trophoblast cells from reactive oxygen species-induced apoptosis in a Bcl-2-dependent manner. Biol Trace Elem Res. 2017;177(2):394–403. https://doi.org/10.1007/s12011-016-0870-5.
Na JY, Seok J, Park S, Kim JS, Kim GJ. Effects of selenium on the survival and invasion of trophoblasts. Clin Exp Reprod Med. 2018;45(1):10–6. https://doi.org/10.5653/cerm.2018.45.1.10.
Habibi N, Labrinidis A, Leemaqz SY, Jankovic-Karasoulos T, McCullough D, Grieger JA, et al. Effect of Selenium and Iodine on Oxidative Stress in the First Trimester Human Placenta Explants. Nutrients. 2021;13(3). https://doi.org/10.3390/nu13030800.
Rayman MP, Searle E, Kelly L, Johnsen S, Bodman-Smith K, Bath SC, et al. Effect of selenium on markers of risk of pre-eclampsia in UK pregnant women: a randomised, controlled pilot trial. Br J Nutr. 2014;112(1):99–111. https://doi.org/10.1017/S0007114514000531.
Tara F, Maamouri G, Rayman MP, Ghayour-Mobarhan M, Sahebkar A, Yazarlu O, et al. Selenium supplementation and the incidence of preeclampsia in pregnant Iranian women: a randomized, double-blind, placebo-controlled pilot trial. Taiwan J Obstet Gynecol. 2010;49(2):181–7. https://doi.org/10.1016/S1028-4559(10)60038-1.
Murphy B, Bhattacharya R, Mukherjee P. Hydrogen sulfide signaling in mitochondria and disease. FASEB J. 2019;33(12):13098–125. https://doi.org/10.1096/fj.201901304R.
Wang K, Ahmad S, Cai M, Rennie J, Fujisawa T, Crispi F, et al. Dysregulation of hydrogen sulfide producing enzyme cystathionine gamma-lyase contributes to maternal hypertension and placental abnormalities in preeclampsia. Circulation. 2013;127(25):2514–22. https://doi.org/10.1161/CIRCULATIONAHA.113.001631.
Saif J, Ahmad S, Rezai H, Litvinova K, Sparatore A, Alzahrani FA, et al. Hydrogen sulfide releasing molecule MZe786 inhibits soluble Flt-1 and prevents preeclampsia in a refined RUPP mouse model. Redox Biol. 2021;38: 101814. https://doi.org/10.1016/j.redox.2020.101814.
Szczesny B, Modis K, Yanagi K, Coletta C, Le Trionnaire S, Perry A, et al. AP39, a novel mitochondria-targeted hydrogen sulfide donor, stimulates cellular bioenergetics, exerts cytoprotective effects and protects against the loss of mitochondrial DNA integrity in oxidatively stressed endothelial cells in vitro. Nitric Oxide. 2014;41:120–30. https://doi.org/10.1016/j.niox.2014.04.008.
Sanchez-Aranguren LC, Ahmad S, Dias IHK, Alzahrani FA, Rezai H, Wang K, et al. Bioenergetic effects of hydrogen sulfide suppress soluble Flt-1 and soluble endoglin in cystathionine gamma-lyase compromised endothelial cells. Sci Rep. 2020;10(1):15810. https://doi.org/10.1038/s41598-020-72371-2.
Libiad M, Vitvitsky V, Bostelaar T, Bak DW, Lee HJ, Sakamoto N, et al. Hydrogen sulfide perturbs mitochondrial bioenergetics and triggers metabolic reprogramming in colon cells. J Biol Chem. 2019;294(32):12077–90. https://doi.org/10.1074/jbc.RA119.009442.
Reiter RJ, Ma Q, Sharma R. Melatonin in mitochondria: mitigating clear and present dangers. Physiology (Bethesda). 2020;35(2):86–95. https://doi.org/10.1152/physiol.00034.2019.
Huo X, Wang C, Yu Z, Peng Y, Wang S, Feng S, et al. Human transporters, PEPT1/2, facilitate melatonin transportation into mitochondria of cancer cells: an implication of the therapeutic potential. J Pineal Res. 2017;62(4). https://doi.org/10.1111/jpi.12390.
Venegas C, Garcia JA, Escames G, Ortiz F, Lopez A, Doerrier C, et al. Extrapineal melatonin: analysis of its subcellular distribution and daily fluctuations. J Pineal Res. 2012;52(2):217–27. https://doi.org/10.1111/j.1600-079X.2011.00931.x.
Hannan NJ, Binder NK, Beard S, Nguyen TV, Kaitu’u-Lino TJ, Tong S. Melatonin enhances antioxidant molecules in the placenta, reduces secretion of soluble fms-like tyrosine kinase 1 (sFLT) from primary trophoblast but does not rescue endothelial dysfunction: An evaluation of its potential to treat preeclampsia. PLoS ONE. 2018;13(4): e0187082. https://doi.org/10.1371/journal.pone.0187082.
Lanoix D, Lacasse AA, Reiter RJ, Vaillancourt C. Melatonin: the watchdog of villous trophoblast homeostasis against hypoxia/reoxygenation-induced oxidative stress and apoptosis. Mol Cell Endocrinol. 2013;381(1–2):35–45. https://doi.org/10.1016/j.mce.2013.07.010.
Nagai R, Watanabe K, Wakatsuki A, Hamada F, Shinohara K, Hayashi Y, et al. Melatonin preserves fetal growth in rats by protecting against ischemia/reperfusion-induced oxidative/nitrosative mitochondrial damage in the placenta. J Pineal Res. 2008;45(3):271–6. https://doi.org/10.1111/j.1600-079X.2008.00586.x.
Hobson SR, Gurusinghe S, Lim R, Alers NO, Miller SL, Kingdom JC, et al. Melatonin improves endothelial function in vitro and prolongs pregnancy in women with early-onset preeclampsia. J Pineal Res. 2018;65(3): e12508. https://doi.org/10.1111/jpi.12508.
Onda K, Tong S, Beard S, Binder N, Muto M, Senadheera SN, et al. Proton pump inhibitors decrease soluble fms-like tyrosine kinase-1 and soluble endoglin secretion, decrease hypertension, and rescue endothelial dysfunction. Hypertension. 2017;69(3):457–68. https://doi.org/10.1161/HYPERTENSIONAHA.116.08408.
Rai K, Matsui H, Kaneko T, Nagano Y, Shimokawa O, Udo J, et al. Lansoprazole inhibits mitochondrial superoxide production and cellular lipid peroxidation induced by indomethacin in RGM1 cells. J Clin Biochem Nutr. 2011;49(1):25–30. https://doi.org/10.3164/jcbn.10-133.
Saleh L, Samantar R, Garrelds IM, van den Meiracker AH, Visser W, Danser AHJ. Low soluble Fms-like tyrosine kinase-1, endoglin, and endothelin-1 levels in women with confirmed or suspected preeclampsia using proton pump inhibitors. Hypertension. 2017;70(3):594–600. https://doi.org/10.1161/HYPERTENSIONAHA.117.09741.
Herzig S, Shaw RJ. AMPK: guardian of metabolism and mitochondrial homeostasis. Nat Rev Mol Cell Biol. 2018;19(2):121–35. https://doi.org/10.1038/nrm.2017.95.
Banek CT, Bauer AJ, Needham KM, Dreyer HC, Gilbert JS. AICAR administration ameliorates hypertension and angiogenic imbalance in a model of preeclampsia in the rat. Am J Physiol Heart Circ Physiol. 2013;304(8):H1159–65. https://doi.org/10.1152/ajpheart.00903.2012.
Lane SL, Houck JA, Doyle AS, Bales ES, Lorca RA, Julian CG, et al. AMP-activated protein kinase activator AICAR attenuates hypoxia-induced murine fetal growth restriction in part by improving uterine artery blood flow. J Physiol. 2020;598(18):4093–105. https://doi.org/10.1113/JP279341.
Landau D, Haghiac M, Minium J, Skomorovska-Prokvolit Y, Calabuig-Navarro V, O’Tierney-Ginn P. Activation of AMPK in human placental explants impairs mitochondrial function and cellular metabolism. Reprod Sci. 2019;26(4):487–95. https://doi.org/10.1177/1933719118776803.
Brownfoot FC, Hastie R, Hannan NJ, Cannon P, Tuohey L, Parry LJ, et al. Metformin as a prevention and treatment for preeclampsia: effects on soluble fms-like tyrosine kinase 1 and soluble endoglin secretion and endothelial dysfunction. Am J Obstet Gynecol. 2016;214(3):356 e1- e15. https://doi.org/10.1016/j.ajog.2015.12.019
Hu J, Zhang J, Zhu B. Protective effect of metformin on a rat model of lipopolysaccharide-induced preeclampsia. Fundam Clin Pharmacol. 2019;33(6):649–58. https://doi.org/10.1111/fcp.12501.
Cluver C, Walker SP, Mol BW, Hall D, Hiscock R, Brownfoot FC, et al. A double blind, randomised, placebo-controlled trial to evaluate the efficacy of metformin to treat preterm pre-eclampsia (PI2 Trial): study protocol. BMJ Open. 2019;9(4): e025809. https://doi.org/10.1136/bmjopen-2018-025809.
Zou Y, Zuo Q, Huang S, Yu X, Jiang Z, Zou S, et al. Resveratrol inhibits trophoblast apoptosis through oxidative stress in preeclampsia-model rats. Molecules. 2014;19(12):20570–9. https://doi.org/10.3390/molecules191220570.
Hannan NJ, Brownfoot FC, Cannon P, Deo M, Beard S, Nguyen TV, et al. Resveratrol inhibits release of soluble fms-like tyrosine kinase (sFlt-1) and soluble endoglin and improves vascular dysfunction-implications as a preeclampsia treatment. Sci Rep. 2017;7(1):1819. https://doi.org/10.1038/s41598-017-01993-w.
Zou Y, Li S, Wu D, Xu Y, Wang S, Jiang Y, et al. Resveratrol promotes trophoblast invasion in pre-eclampsia by inducing epithelial-mesenchymal transition. J Cell Mol Med. 2019;23(4):2702–10. https://doi.org/10.1111/jcmm.14175.
Handschin C, Spiegelman BM. Peroxisome proliferator-activated receptor gamma coactivator 1 coactivators, energy homeostasis, and metabolism. Endocr Rev. 2006;27(7):728–35. https://doi.org/10.1210/er.2006-0037.
Lane SL, Dodson RB, Doyle AS, Park H, Rathi H, Matarrazo CJ, et al. Pharmacological activation of peroxisome proliferator-activated receptor gamma (PPAR-gamma) protects against hypoxia-associated fetal growth restriction. FASEB J. 2019;33(8):8999–9007. https://doi.org/10.1096/fj.201900214R.
McCarthy FP, Drewlo S, Kingdom J, Johns EJ, Walsh SK, Kenny LC. Peroxisome proliferator-activated receptor-gamma as a potential therapeutic target in the treatment of preeclampsia. Hypertension. 2011;58(2):280–6. https://doi.org/10.1161/HYPERTENSIONAHA.111.172627.
Funding
This work was funded by the National Institutes of Health Grants HD083132 (LZ), HL128209 (LZ), HL137649 (LZ) and HL149608 (LZ).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflicts of Interest
The authors declare no conflict of interest.
Human and Animal Rights and Informed
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Preeclampsia
Rights and permissions
About this article
Cite this article
Hu, XQ., Zhang, L. Mitochondrial Dysfunction in the Pathogenesis of Preeclampsia. Curr Hypertens Rep 24, 157–172 (2022). https://doi.org/10.1007/s11906-022-01184-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11906-022-01184-7