Abstract
The nitric oxide (NO)-cyclic guanosine monophosphate (cGMP) signaling system is a well-characterized modulator of cardiovascular function, in general, and blood pressure, in particular. The availability of mice mutant for key enzymes in the NO-cGMP signaling system facilitated the identification of interactions with other blood pressure modifying pathways (e.g. the renin-angiotensin-aldosterone system) and of gender-specific effects of impaired NO-cGMP signaling. In addition, recent genome-wide association studies identified blood pressure-modifying genetic variants in genes that modulate NO and cGMP levels. Together, these findings have advanced our understanding of how NO-cGMP signaling regulates blood pressure. In this review, we will summarize the results obtained in mice with disrupted NO-cGMP signaling and highlight the relevance of this pathway as a potential therapeutic target for the treatment of hypertension.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
• Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, et al. Executive Summary: Heart Disease and Stroke Statistics–2012 Update: A Report From the American Heart Association. Circulation. 2012;125(1):188–97. doi:10.1161/CIR.0b013e3182456d46. The 2012 AHA Statistical Update, a comprehensive overview of cardiovascular health and disease in the population, illustrates how big a burden on society uncontrolled hypertension is.
Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo Jr JL, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42(6):1206–52. doi:10.1161/01.HYP.0000107251.49515.c2.
Kung H-C, Hoyert DL, Xu J, Murphy SL. Deaths: Final Data for 2005. Natl Vital Stat Rep. 2008;56(10):1–121.
Lifton RP, Gharavi AG, Geller DS. Molecular mechanisms of human hypertension. Cell. 2001;104(4):545–56.
Newton-Cheh C, Johnson T, Gateva V, Tobin MD, Bochud M, Coin L, et al. Genome-wide association study identifies eight loci associated with blood pressure. Nat Genet. 2009;41(6):666–76. doi:10.1038/ng.361.
Hamm LL, Hering-Smith KS. Pivotal role of the kidney in hypertension. Am J Med Sci. 2010;340(1):30–2. doi:10.1097/MAJ.0b013e3181e590f0.
Ruilope LM. Hypertension in 2010: Blood pressure and the kidney. Nat Rev Nephrol. 2011;7(2):73–4. doi:10.1038/nrneph.2010.189.
Taylor RS, Ashton KE, Moxham T, Hooper L, Ebrahim S. Reduced dietary salt for the prevention of cardiovascular disease: a meta-analysis of randomized controlled trials (cochrane review). Am J Hypertens. 2011;24(8):843–53. doi:10.1038/ajh.2011.115.
Mendelsohn ME. In hypertension, the kidney is not always the heart of the matter. J Clin Invest. 2005;115(4):840–4.
•• Buys ES, Raher MJ, Kirby A, Mohd S, Baron DM, Hayton SR, et al. Genetic modifiers of hypertension in soluble guanylate cyclase alpha1-deficient mice. J Clin Invest. 2012;122(6):2316–25. doi:10.1172/JCI60119. Studies in sGC mutant mice illustrate that sGC signaling has gender-specific cardiovascular effects, identified quantitative trait loci linked to mean arterial pressure (MAP) in the context of sGC deficiency, and identified the RAAS as a blood pressure-modifying mechanism in a setting of impaired NO/cGMP signaling.
• Michael SK, Surks HK, Wang Y, Zhu Y, Blanton R, Jamnongjit M, et al. High blood pressure arising from a defect in vascular function. Proc Natl Acad Sci USA. 2008;105(18):6702–7. In this study it was shown that mice with a mutant PKGI develop hypertension due to dysfunctional NO-induced vascular relaxation. This observation confirms that hypertension can arise due to a primary vascular defect.
Friebe A, Mergia E, Dangel O, Lange A, Koesling D. Fatal gastrointestinal obstruction and hypertension in mice lacking nitric oxide-sensitive guanylyl cyclase. Proc Natl Acad Sci USA. 2007;104(18):7699–704. doi:10.1073/pnas.0609778104.
Moncada S, Higgs A. The L-arginine-nitric oxide pathway. N Engl J Med. 1993;329(27):2002–12.
Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM. Activation of nitric oxide synthase in endothelial cells by Akt- dependent phosphorylation. Nature. 1999;399(6736):601–5.
Lundberg JO, Weitzberg E, Gladwin MT. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat Rev Drug Discov. 2008;7(2):156–67. doi:10.1038/nrd2466.
Stamler JS, Lamas S, Fang FC. Nitrosylation. the prototypic redox-based signaling mechanism. Cell. 2001;106(6):675–83.
Davis KL, Martin E, Turko IV, Murad F. Novel effects of nitric oxide. Annu Rev Pharmacol Toxicol. 2001;41:203–36.
Derbyshire ER, Marletta MA. Structure and regulation of soluble guanylate cyclase. Annu Rev Biochem. 2012;81:533–59. doi:10.1146/annurev-biochem-050410-100030.
Hobbs AJ. Soluble guanylate cyclase: the forgotten sibling. Trends Pharmacol Sci. 1997;18(12):484–91.
Wedel B, Humbert P, Harteneck C, Foerster J, Malkewitz J, Bohme E, et al. Mutation of His-105 in the beta 1 subunit yields a nitric oxide-insensitive form of soluble guanylyl cyclase. Proc Natl Acad Sci USA. 1994;91(7):2592–6.
Mergia E, Russwurm M, Zoidl G, Koesling D. Major occurrence of the new alpha(2)beta(1) isoform of NO-sensitive guanylyl cyclase in brain. Cell Signal. 2003;15(2):189–95.
• Buys ES, Sips P, Vermeersch P, Raher MJ, Rogge E, Ichinose F, et al. Gender-specific hypertension and responsiveness to nitric oxide in sGC{alpha}1 knockout mice. Cardiovasc Res. 2008;79(1):179–86. This report indicated that loss of sGCα1 has a gender-specific effect on blood pressure, while showing that even low levels of sGC activation are still sufficient to produce NO-dependent vasorelaxation.
Nimmegeers S, Sips P, Buys E, Brouckaert P, Van de Voorde J. Functional role of the soluble guanylyl cyclase alpha(1) subunit in vascular smooth muscle relaxation. Cardiovasc Res. 2007;76(1):149–59.
Vermeersch P, Buys E, Pokreisz P, Marsboom G, Ichinose F, Sips P, et al. Soluble guanylate cyclase-alpha1 deficiency selectively inhibits the pulmonary vasodilator response to nitric oxide and increases the pulmonary vascular remodeling response to chronic hypoxia. Circulation. 2007;116(8):936–43.
Mergia E, Friebe A, Dangel O, Russwurm M, Koesling D. Spare guanylyl cyclase NO receptors ensure high NO sensitivity in the vascular system. J Clin Invest. 2006;116(6):1731–7. doi:10.1172/JCI27657.
Stauss HM, Godecke A, Mrowka R, Schrader J, Persson PB. Enhanced blood pressure variability in eNOS knockout mice. Hypertension. 1999;33(6):1359–63.
Sips PY, Brouckaert P, Ichinose F. The alpha1 isoform of soluble guanylate cyclase regulates cardiac contractility but is not required for ischemic preconditioning. Basic Res Cardiol. 2011;106(4):635–43. doi:10.1007/s00395-011-0167-y.
Garbers DL, Chrisman TD, Wiegn P, Katafuchi T, Albanesi JP, Bielinski V, et al. Membrane guanylyl cyclase receptors: an update. Trends Endocrinol Metab. 2006;17(6):251–8. doi:10.1016/j.tem.2006.06.006.
Su J, Scholz PM, Weiss HR. Differential effects of cGMP produced by soluble and particulate guanylyl cyclase on mouse ventricular myocytes. Exp Biol Med (Maywood). 2005;230(4):242–50.
Castro LR, Verde I, Cooper DM, Fischmeister R. Cyclic guanosine monophosphate compartmentation in rat cardiac myocytes. Circulation. 2006;113(18):2221–8.
Piggott LA, Hassell KA, Berkova Z, Morris AP, Silberbach M, Rich TC. Natriuretic peptides and nitric oxide stimulate cGMP synthesis in different cellular compartments. J Gen Physiol. 2006;128(1):3–14.
•• Ehret GB, Munroe PB, Rice KM, Bochud M, Johnson AD, Chasman DI, et al. Genetic variants in novel pathways influence blood pressure and cardiovascular disease risk. Nature. 2011;478(7367):103–9. doi:10.1038/nature10405. This genome-wide association study of systolic and diastolic blood pressure identified sixteen novel loci in 200,000 individuals of European descent, including a variant in the GUCY1A3/GUCY1B3 locus, encoding the sGCα1 and sGCβ1 subunits. This study provides new insights into the genetics and biology of blood pressure, and suggest potential novel therapeutic pathways for cardiovascular disease prevention.
Panza JA, Quyyumi AA, Brush Jr JE, Epstein SE. Abnormal endothelium-dependent vascular relaxation in patients with essential hypertension. N Engl J Med. 1990;323(1):22–7. doi:10.1056/NEJM199007053230105.
Friebe A, Koesling D. The function of NO-sensitive guanylyl cyclase: what we can learn from genetic mouse models. Nitric Oxide. 2009;21(3–4):149–56.
Moncada S, Higgs EA. Nitric oxide and the vascular endothelium. Handb Exp Pharmacol. 2006;176(Pt 1):213–54.
Rapoport RM, Murad F. Agonist-induced endothelium-dependent relaxation in rat thoracic aorta may be mediated through cGMP. Circ Res. 1983;52(3):352–7.
Ignarro LJ. Endothelium-derived nitric oxide: actions and properties. FASEB J. 1989;3(1):31–6.
Huang PL, Huang Z, Mashimo H, Bloch KD, Moskowitz MA, Bevan JA, et al. Hypertension in mice lacking the gene for endothelial nitric oxide synthase. Nature. 1995;377(6546):239–42.
Shesely EG, Maeda N, Kim HS, Desai KM, Krege JH, Laubach VE, et al. Elevated blood pressures in mice lacking endothelial nitric oxide synthase. Proc Natl Acad Sci USA. 1996;93(23):13176–81.
Beierwaltes WH, Potter DL, Shesely EG. Renal baroreceptor-stimulated renin in the eNOS knockout mouse. Am J Physiol Renal Physiol. 2002;282(1):F59–64. doi:10.1152/ajprenal.00144.2001.
Kurihara N, Alfie ME, Sigmon DH, Rhaleb NE, Shesely EG, Carretero OA. Role of nNOS in blood pressure regulation in eNOS null mutant mice. Hypertension. 1998;32(5):856–61.
Duplain H, Burcelin RM, Sartori C, Cook SP, Egli M, Lepori M, et al. Insulin Resistance, Hyperlipidemia, and Hypertension in Mice Lacking Endothelial Nitric Oxide Synthase. Circulation. 2001;104(3):342–5. doi:10.1161/01.cir.104.3.342.
Yang X-P, Liu Y-H, Shesely EG, Bulagannawar M, Liu F, Carretero OA. Endothelial Nitric Oxide Gene Knockout Mice. Hypertension. 1999;34(1):24–30. doi:10.1161/01.hyp.34.1.24.
Nelson RJ, Demas GE, Huang PL, Fishman MC, Dawson VL, Dawson TM, et al. Behavioural abnormalities in male mice lacking neuronal nitric oxide synthase. Nature. 1995;378(6555):383–6.
Sun Y, Carretero OA, Xu J, Rhaleb N-E, Yang JJ, Pagano PJ, et al. Deletion of Inducible Nitric Oxide Synthase Provides Cardioprotection in Mice With 2-Kidney, 1-Clip Hypertension. Hypertension. 2009;53(1):49–56. doi:10.1161/hypertensionaha.108.121822.
Nakata S, Tsutsui M, Shimokawa H, Suda O, Morishita T, Shibata K, et al. Spontaneous myocardial infarction in mice lacking all nitric oxide synthase isoforms. Circulation. 2008;117(17):2211–23. doi:10.1161/CIRCULATIONAHA.107.742692.
Barouch LA, Cappola TP, Harrison RW, Crone JK, Rodriguez ER, Burnett AL, et al. Combined loss of neuronal and endothelial nitric oxide synthase causes premature mortality and age-related hypertrophic cardiac remodeling in mice. J Mol Cell Cardiol. 2003;35(6):637–44.
Lamping KG, Faraci FM. Role of sex differences and effects of endothelial NO synthase deficiency in responses of carotid arteries to serotonin. Arterioscler Thromb Vasc Biol. 2001;21(4):523–8.
Scotland RS, Morales-Ruiz M, Chen Y, Yu J, Rudic RD, Fulton D, et al. Functional reconstitution of endothelial nitric oxide synthase reveals the importance of serine 1179 in endothelium-dependent vasomotion. Circ Res. 2002;90(8):904–10.
Ding H, Kubes P, Triggle C. Potassium- and acetylcholine-induced vasorelaxation in mice lacking endothelial nitric oxide synthase. Br J Pharmacol. 2000;129(6):1194–200. doi:10.1038/sj.bjp.0703144.
Scotland RS, Chauhan S, Vallance PJ, Ahluwalia A. An endothelium-derived hyperpolarizing factor-like factor moderates myogenic constriction of mesenteric resistance arteries in the absence of endothelial nitric oxide synthase-derived nitric oxide. Hypertension. 2001;38(4):833–9.
Atochin DN, Yuzawa I, Li Q, Rauwerdink KM, Malhotra R, Chang J, et al. Soluble guanylate cyclase alpha1beta1 limits stroke size and attenuates neurological injury. Stroke. 2010;41(8):1815–9. doi:10.1161/STROKEAHA.109.577635.
Buys ES, Cauwels A, Raher MJ, Passeri JJ, Hobai I, Cawley SM, et al. sGC(alpha)1(beta)1 attenuates cardiac dysfunction and mortality in murine inflammatory shock models. Am J Physiol Heart Circ Physiol. 2009;297(2):H654–63. doi:10.1152/ajpheart.00367.2009.
Thoonen R, Buys E, Cauwels A, Rogge E, Nimmegeers S, Hemel M, et al. NO-insensitive sGCbeta1 H105F knockin mice: if NO has no place to go. BMC Pharmacol. 2009;9 Suppl 1:S41.
Weber S, Bernhard D, Lukowski R, Weinmeister P, Worner R, Wegener JW, et al. Rescue of cGMP kinase I knockout mice by smooth muscle specific expression of either isozyme. Circ Res. 2007;101(11):1096–103. doi:10.1161/CIRCRESAHA.107.154351.
Feil R, Gappa N, Rutz M, Schlossmann J, Rose CR, Konnerth A, et al. Functional reconstitution of vascular smooth muscle cells with cGMP-dependent protein kinase I isoforms. Circ Res. 2002;90(10):1080–6.
Pfeifer A, Klatt P, Massberg S, Ny L, Sausbier M, Hirneiss C, et al. Defective smooth muscle regulation in cGMP kinase I-deficient mice. EMBO J. 1998;17(11):3045–51. doi:10.1093/emboj/17.11.3045.
Koeppen M, Feil R, Siegl D, Feil S, Hofmann F, Pohl U, et al. cGMP-dependent protein kinase mediates NO- but not acetylcholine-induced dilations in resistance vessels in vivo. Hypertension. 2004;44(6):952–5.
Sausbier M, Schubert R, Voigt V, Hirneiss C, Pfeifer A, Korth M, et al. Mechanisms of NO/cGMP-dependent vasorelaxation. Circ Res. 2000;87(9):825–30.
Tang KM, Wang GR, Lu P, Karas RH, Aronovitz M, Heximer SP, et al. Regulator of G-protein signaling-2 mediates vascular smooth muscle relaxation and blood pressure. Nat Med. 2003;9(12):1506–12. doi:10.1038/nm958.
Prysyazhna O, Rudyk O, Eaton P. Single atom substitution in mouse protein kinase G eliminates oxidant sensing to cause hypertension. Nat Med. 2012;18(2):286–90. doi:10.1038/nm.2603.
Xia C, Bao Z, Yue C, Sanborn BM, Liu M. Phosphorylation and regulation of G-protein-activated phospholipase C-beta 3 by cGMP-dependent protein kinases. J Biol Chem. 2001;276(23):19770–7.
Schlossmann J, Ammendola A, Ashman K, Zong X, Huber A, Neubauer G, et al. Regulation of intracellular calcium by a signalling complex of IRAG, IP3 receptor and cGMP kinase Ibeta. Nature. 2000;404(6774):197–201.
Cornwell TL, Pryzwansky KB, Wyatt TA, Lincoln TM. Regulation of sarcoplasmic reticulum protein phosphorylation by localized cyclic GMP-dependent protein kinase in vascular smooth muscle cells. Mol Pharmacol. 1991;40(6):923–31.
Liu H, Xiong Z, Sperelakis N. Cyclic nucleotides regulate the activity of L-type calcium channels in smooth muscle cells from rat portal vein. J Mol Cell Cardiol. 1997;29(5):1411–21.
Archer SL, Huang JM, Hampl V, Nelson DP, Shultz PJ, Weir EK. Nitric oxide and cGMP cause vasorelaxation by activation of a charybdotoxin-sensitive K channel by cGMP-dependent protein kinase. Proc Natl Acad Sci USA. 1994;91(16):7583–7.
Murphy ME, Brayden JE. Nitric oxide hyperpolarizes rabbit mesenteric arteries via ATP-sensitive potassium channels. J Physiol. 1995;486(Pt 1):47–58.
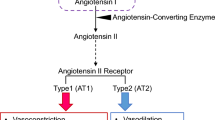
Kobori H, Nangaku M, Navar LG, Nishiyama A. The intrarenal renin-angiotensin system: from physiology to the pathobiology of hypertension and kidney disease. Pharmacol Rev. 2007;59(3):251–87. doi:10.1124/pr.59.3.3.
Hofmann F, Feil R, Kleppisch T, Schlossmann J. Function of cGMP-dependent protein kinases as revealed by gene deletion. Physiol Rev. 2006;86(1):1–23.
Takemoto M, Egashira K, Usui M, Numaguchi K, Tomita H, Tsutsui H, et al. Important role of tissue angiotensin-converting enzyme activity in the pathogenesis of coronary vascular and myocardial structural changes induced by long-term blockade of nitric oxide synthesis in rats. J Clin Invest. 1997;99(2):278–87.
Ichiki T, Usui M, Kato M, Funakoshi Y, Ito K, Egashira K, et al. Downregulation of angiotensin II type 1 receptor gene transcription by nitric oxide. Hypertension. 1998;31(1 Pt 2):342–8.
Usui M, Ichiki T, Katoh M, Egashira K, Takeshita A. Regulation of angiotensin II receptor expression by nitric oxide in rat adrenal gland. Hypertension. 1998;32(3):527–33.
Beierwaltes WH. cGMP stimulates renin secretion in vivo by inhibiting phosphodiesterase-3. Am J Physiol Renal Physiol. 2006;290(6):F1376–81.
Gambaryan S, Butt E, Marcus K, Glazova M, Palmetshofer A, Guillon G, et al. cGMP-dependent protein kinase type II regulates basal level of aldosterone production by zona glomerulosa cells without increasing expression of the steroidogenic acute regulatory protein gene. J Biol Chem. 2003;278(32):29640–8.
Johnson AD, Newton-Cheh C, Chasman DI, Ehret GB, Johnson T, Rose L, et al. Association of Hypertension Drug Target Genes With Blood Pressure and Hypertension in 86 588 Individuals. Hypertension. 2011;57(5):903–10. doi:10.1161/HYPERTENSIONAHA.110.158667.
Kato N, Takeuchi F, Tabara Y, Kelly TN, Go MJ, Sim X, et al. Meta-analysis of genome-wide association studies identifies common variants associated with blood pressure variation in east Asians. Nat Genet. 2011;43(6):531–8. doi:10.1038/ng.834.
Levy D, Ehret GB, Rice K, Verwoert GC, Launer LJ, Dehghan A, et al. Genome-wide association study of blood pressure and hypertension. Nat Genet. 2009;41(6):677–87. doi:10.1038/ng.384.
Newton-Cheh C, Larson MG, Vasan RS, Levy D, Bloch KD, Surti A, et al. Association of common variants in NPPA and NPPB with circulating natriuretic peptides and blood pressure. Nat Genet. 2009;41(3):348–53. doi:10.1038/ng.328.
Johnson T, Gaunt TR, Newhouse SJ, Padmanabhan S, Tomaszewski M, Kumari M, et al. Blood pressure loci identified with a gene-centric array. Am J Hum Genet. 2011;89(6):688–700. doi:10.1016/j.ajhg.2011.10.013.
Kathiresan S, Larson MG, Vasan RS, Guo CY, Vita JA, Mitchell GF, et al. Common genetic variation at the endothelial nitric oxide synthase locus and relations to brachial artery vasodilator function in the community. Circulation. 2005;112(10):1419–27. doi:10.1161/CIRCULATIONAHA.105.544619.
Mitchell GF, Guo CY, Kathiresan S, Vasan RS, Larson MG, Vita JA, et al. Vascular stiffness and genetic variation at the endothelial nitric oxide synthase locus: the Framingham Heart study. Hypertension. 2007;49(6):1285–90. doi:10.1161/HYPERTENSIONAHA.106.085266.
Salvi E, Kutalik Z, Glorioso N, Benaglio P, Frau F, Kuznetsova T, et al. Genomewide association study using a high-density single nucleotide polymorphism array and case–control design identifies a novel essential hypertension susceptibility locus in the promoter region of endothelial NO synthase. Hypertension. 2012;59(2):248–55. doi:10.1161/HYPERTENSIONAHA.111.181990.
Fedorowski A, Franceschini N, Brody J, Liu C, Verwoert GC, Boerwinkle E, et al. Orthostatic hypotension and novel blood pressure-associated gene variants: Genetics of Postural Hemodynamics (GPH) Consortium. Eur Heart J. 2012. doi:10.1093/eurheartj/ehs058.
Zhu X, Young JH, Fox E, Keating BJ, Franceschini N, Kang S, et al. Combined admixture mapping and association analysis identifies a novel blood pressure genetic locus on 5p13: contributions from the CARe consortium. Hum Mol Genet. 2011;20(11):2285–95. doi:10.1093/hmg/ddr113.
Kannel WB WP, Garrison RJ. Some risk factors related to the annual incidence of cardiovascular disease and death using pooled repeated biennial measurements: Framingham Heart Study, 30 year followup. Springfield: National Technical Information Service; 1987.
Burt VL, Whelton P, Roccella EJ, Brown C, Cutler JA, Higgins M, et al. Prevalence of hypertension in the US adult population. Results from the Third National Health and Nutrition Examination Survey, 1988–1991. Hypertension. 1995;25(3):305–13.
Reckelhoff JF. Gender differences in the regulation of blood pressure. Hypertension. 2001;37(5):1199–208.
Scotland RS, Madhani M, Chauhan S, Moncada S, Andresen J, Nilsson H, et al. Investigation of vascular responses in endothelial nitric oxide synthase/cyclooxygenase-1 double-knockout mice: key role for endothelium-derived hyperpolarizing factor in the regulation of blood pressure in vivo. Circulation. 2005;111(6):796–803.
Beigi F, Gonzalez DR, Minhas KM, Sun QA, Foster MW, Khan SA, et al. Dynamic denitrosylation via S-nitrosoglutathione reductase regulates cardiovascular function. Proc Natl Acad Sci USA. 2012;109(11):4314–9. doi:10.1073/pnas.1113319109.
Liu L, Yan Y, Zeng M, Zhang J, Hanes MA, Ahearn G, et al. Essential roles of S-nitrosothiols in vascular homeostasis and endotoxic shock. Cell. 2004;116(4):617–28.
Sanghani PC, Davis WI, Fears SL, Green SL, Zhai L, Tang Y, et al. Kinetic and cellular characterization of novel inhibitors of S-nitrosoglutathione reductase. J Biol Chem. 2009;284(36):24354–62. doi:10.1074/jbc.M109.019919.
Greco TM, Hodara R, Parastatidis I, Heijnen HF, Dennehy MK, Liebler DC, et al. Identification of S-nitrosylation motifs by site-specific mapping of the S-nitrosocysteine proteome in human vascular smooth muscle cells. Proc Natl Acad Sci USA. 2006;103(19):7420–5. doi:10.1073/pnas.0600729103.
Ulrich C, Quillici DR, Schegg K, Woolsey R, Nordmeier A, Buxton IL. Uterine smooth muscle S-nitrosylproteome in pregnancy. Mol Pharmacol. 2012;81(2):143–53. doi:10.1124/mol.111.075804.
Lang RJ, Harvey JR, McPhee GJ, Klemm MF. Nitric oxide and thiol reagent modulation of Ca2 + −activated K + (BKCa) channels in myocytes of the guinea-pig taenia caeci. J Physiol. 2000;525(Pt 2):363–76.
Bolotina VM, Najibi S, Palacino JJ, Pagano PJ, Cohen RA. Nitric oxide directly activates calcium-dependent potassium channels in vascular smooth muscle. Nature. 1994;368(6474):850–3.
Sayed N, Baskaran P, Ma X, van den Akker F, Beuve A. Desensitization of soluble guanylyl cyclase, the NO receptor, by S-nitrosylation. Proc Natl Acad Sci USA. 2007;104(30):12312–7. doi:10.1073/pnas.0703944104.
• Stasch JP, Schmidt PM, Nedvetsky PI, Nedvetskaya TY, Arum Kumar HS, Meurer S, et al. Targeting the heme-oxidized nitric oxide receptor for selective vasodilatation of diseased blood vessels. J Clin Invest. 2006;116(9):2552–61. This study proved for the first time the pharmacological principle of sGC activators under conditions of increased oxidative stress.
Touyz RM. Reactive oxygen species, vascular oxidative stress, and redox signaling in hypertension: what is the clinical significance? Hypertension. 2004;44(3):248–52. doi:10.1161/01.HYP.0000138070.47616.9d.
Chirkov YY, Horowitz JD. Impaired tissue responsiveness to organic nitrates and nitric oxide: a new therapeutic frontier? Pharmacol Ther. 2007;116(2):287–305. doi:10.1016/j.pharmthera.2007.06.012.
Franco V, Oparil S. Is there a new treatment for hypertensive disease in the horizon? Role of soluble guanylate cyclase. Hypertension. 2006;48(5):822–3.
Foerster J, Harteneck C, Malkewitz J, Schultz G, Koesling D. A functional heme-binding site of soluble guanylyl cyclase requires intact N-termini of alpha 1 and beta 1 subunits. Eur J Biochem. 1996;240(2):380–6.
Zhao Y, Brandish PE, DiValentin M, Schelvis JP, Babcock GT, Marletta MA. Inhibition of soluble guanylate cyclase by ODQ. Biochemistry. 2000;39(35):10848–54.
Ahrens I, Habersberger J, Baumlin N, Qian H, Smith BK, Stasch JP, et al. Measuring oxidative burden and predicting pharmacological response in coronary artery disease patients with a novel direct activator of haem-free/oxidised sGC. Atherosclerosis. 2011;218(2):431–4. doi:10.1016/j.atherosclerosis.2011.06.042.
Evgenov OV, Pacher P, Schmidt PM, Hasko G, Schmidt HH, Stasch JP. NO-independent stimulators and activators of soluble guanylate cyclase: discovery and therapeutic potential. Nat Rev Drug Discov. 2006;5(9):755–68.
Doggrell SA. Clinical potential of nitric oxide-independent soluble guanylate cyclase activators. Curr Opin Investig Drugs. 2005;6(9):874–8.
Stasch JP, Dembowsky K, Perzborn E, Stahl E, Schramm M. Cardiovascular actions of a novel NO-independent guanylyl cyclase stimulator, BAY 41–8543: in vivo studies. Br J Pharmacol. 2002;135(2):344–55.
Straub A, Benet-Buckholz J, Frode R, Kern A, Kohlsdorfer C, Schmitt P, et al. Metabolites of orally active NO-independent pyrazolopyridine stimulators of soluble guanylate cyclase. Bioorg Med Chem. 2002;10(6):1711–7.
Badejo Jr AM, Nossaman VE, Pankey EA, Bhartiya M, Kannadka CB, Murthy SN, et al. Pulmonary and systemic vasodilator responses to the soluble guanylyl cyclase stimulator, BAY 41–8543, are modulated by nitric oxide. Am J Physiol Heart Circ Physiol. 2010;299(4):H1153–9. doi:10.1152/ajpheart.01101.2009.
Stasch JP, Becker EM, Alonso-Alija C, Apeler H, Dembowsky K, Feurer A, et al. NO-independent regulatory site on soluble guanylate cyclase. Nature. 2001;410(6825):212–5. doi:10.1038/35065611.
Masuyama H, Tsuruda T, Kato J, Imamura T, Asada Y, Stasch JP, et al. Soluble guanylate cyclase stimulation on cardiovascular remodeling in angiotensin II-induced hypertensive rats. Hypertension. 2006;48(5):972–8.
Zanfolin M, Faro R, Araujo EG, Guaraldo AM, Antunes E, De Nucci G. Protective effects of BAY 41–2272 (sGC stimulator) on hypertension, heart, and cardiomyocyte hypertrophy induced by chronic L-NAME treatment in rats. J Cardiovasc Pharmacol. 2006;47(3):391–5.
Stasch JP, Alonso-Alija C, Apeler H, Dembowsky K, Feurer A, Minuth T, et al. Pharmacological actions of a novel NO-independent guanylyl cyclase stimulator, BAY 41–8543: in vitro studies. Br J Pharmacol. 2002;135(2):333–43. doi:10.1038/sj.bjp.0704484.
Dumitrascu R, Weissmann N, Ghofrani HA, Dony E, Beuerlein K, Schmidt H, et al. Activation of soluble guanylate cyclase reverses experimental pulmonary hypertension and vascular remodeling. Circulation. 2006;113(2):286–95. doi:10.1161/CIRCULATIONAHA.105.581405.
Boerrigter G, Costello-Boerrigter LC, Cataliotti A, Lapp H, Stasch JP, Burnett Jr JC. Targeting heme-oxidized soluble guanylate cyclase in experimental heart failure. Hypertension. 2007;49(5):1128–33. doi:10.1161/HYPERTENSIONAHA.106.083832.
Oberwittler H, Hirschfeld-Warneken A, Wesch R, Willerich H, Teichert L, Lehr KH, et al. Significant pharmacokinetic and pharmacodynamic interaction of warfarin with the NO-independent sGC activator HMR1766. J Clin Pharmacol. 2007;47(1):70–7. doi:10.1177/0091270006294540.
Benz K, Orth SR, Simonaviciene A, Linz W, Schindler U, Rutten H, et al. Blood pressure-independent effect of long-term treatment with the soluble heme-independent guanylyl cyclase activator HMR1766 on progression in a model of noninflammatory chronic renal damage. Kidney Blood Press Res. 2007;30(4):224–33. doi:10.1159/000104091.
Stasch JP, Schmidt P, Alonso-Alija C, Apeler H, Dembowsky K, Haerter M, et al. NO- and haem-independent activation of soluble guanylyl cyclase: molecular basis and cardiovascular implications of a new pharmacological principle. Br J Pharmacol. 2002;136(5):773–83. doi:10.1038/sj.bjp.0704778.
Knorr A, Hirth-Dietrich C, Alonso-Alija C, Harter M, Hahn M, Keim Y, et al. Nitric oxide-independent activation of soluble guanylate cyclase by BAY 60–2770 in experimental liver fibrosis. Arzneimittelforschung. 2008;58(2):71–80. doi:10.1055/s-0031-1296471.
Pankey EA, Bhartiya M, Badejo Jr AM, Haider U, Stasch JP, Murthy SN, et al. Pulmonary and systemic vasodilator responses to the soluble guanylyl cyclase activator, BAY 60–2770, are not dependent on endogenous nitric oxide or reduced heme. Am J Physiol Heart Circ Physiol. 2011;300(3):H792–802. doi:10.1152/ajpheart.00953.2010.
Korkmaz S, Radovits T, Barnucz E, Hirschberg K, Neugebauer P, Loganathan S, et al. Pharmacological activation of soluble guanylate cyclase protects the heart against ischemic injury. Circulation. 2009;120(8):677–86. doi:10.1161/CIRCULATIONAHA.109.870774.
Radovits T, Korkmaz S, Miesel-Groschel C, Seidel B, Stasch JP, Merkely B, et al. Pre-conditioning with the soluble guanylate cyclase activator Cinaciguat reduces ischaemia-reperfusion injury after cardiopulmonary bypass. Eur J Cardiothorac Surg. 2011;39(2):248–55. doi:10.1016/j.ejcts.2010.05.025.
Frey R, Muck W, Unger S, Artmeier-Brandt U, Weimann G, Wensing G. Pharmacokinetics, pharmacodynamics, tolerability, and safety of the soluble guanylate cyclase activator cinaciguat (BAY 58–2667) in healthy male volunteers. J Clin Pharmacol. 2008;48(12):1400–10. doi:10.1177/0091270008322906.
• Lapp H, Mitrovic V, Franz N, Heuer H, Buerke M, Wolfertz J, et al. Cinaciguat (BAY 58–2667) improves cardiopulmonary hemodynamics in patients with acute decompensated heart failure. Circulation. 2009;119(21):2781–8. doi:10.1161/CIRCULATIONAHA.108.800292. This clinical study showed that cinaciguat reduces systemic vascular resistance in patients with heart failure, demonstrating the proof of concept that specific activation of oxidized sGC has the expected hemodynamic effects in patients.
Huang PL, Dawson TM, Bredt DS, Snyder SH, Fishman MC. Targeted disruption of the neuronal nitric oxide synthase gene. Cell. 1993;75(7):1273–86.
Gyurko R, Leupen S, Huang PL. Deletion of exon 6 of the neuronal nitric oxide synthase gene in mice results in hypogonadism and infertility. Endocrinology. 2002;143(7):2767–74.
Godecke A, Decking UK, Ding Z, Hirchenhain J, Bidmon HJ, Godecke S, et al. Coronary hemodynamics in endothelial NO synthase knockout mice. Circ Res. 1998;82(2):186–94.
Gregg AR, Schauer A, Shi O, Liu Z, Lee CG, O'Brien WE. Limb reduction defects in endothelial nitric oxide synthase-deficient mice. Am J Physiol. 1998;275(6 Pt 2):H2319–24.
Wei XQ, Charles IG, Smith A, Ure J, Feng GJ, Huang FP, et al. Altered immune responses in mice lacking inducible nitric oxide synthase. Nature. 1995;375(6530):408–11. doi:10.1038/375408a0.
Laubach VE, Shesely EG, Smithies O, Sherman PA. Mice lacking inducible nitric oxide synthase are not resistant to lipopolysaccharide-induced death. Proc Natl Acad Sci USA. 1995;92(23):10688–92.
MacMicking JD, Nathan C, Hom G, Chartrain N, Fletcher DS, Trumbauer M, et al. Altered responses to bacterial infection and endotoxic shock in mice lacking inducible nitric oxide synthase. Cell. 1995;81(4):641–50.
Tranguch S, Huet-Hudson Y. Decreased viability of nitric oxide synthase double knockout mice. Mol Reprod Dev. 2003;65(2):175–9. doi:10.1002/mrd.10274.
Morishita T, Tsutsui M, Shimokawa H, Sabanai K, Tasaki H, Suda O, et al. Nephrogenic diabetes insipidus in mice lacking all nitric oxide synthase isoforms. Proc Natl Acad Sci USA. 2005;102(30):10616–21. doi:10.1073/pnas.0502236102.
Son H, Hawkins RD, Martin K, Kiebler M, Huang PL, Fishman MC, et al. Long-term potentiation is reduced in mice that are doubly mutant in endothelial and neuronal nitric oxide synthase. Cell. 1996;87(6):1015–23.
Burkard N, Rokita AG, Kaufmann SG, Hallhuber M, Wu R, Hu K, et al. Conditional neuronal nitric oxide synthase overexpression impairs myocardial contractility. Circ Res. 2007;100(3):e32–44. doi:10.1161/01.RES.0000259042.04576.6a.
Loyer X, Gomez AM, Milliez P, Fernandez-Velasco M, Vangheluwe P, Vinet L, et al. Cardiomyocyte overexpression of neuronal nitric oxide synthase delays transition toward heart failure in response to pressure overload by preserving calcium cycling. Circulation. 2008;117(25):3187–98. doi:10.1161/CIRCULATIONAHA.107.741702.
Packer MA, Hemish J, Mignone JL, John S, Pugach I, Enikolopov G. Transgenic mice overexpressing nNOS in the adult nervous system. Cell Mol Biol (Noisy-le-Grand). 2005;51(3):269–77.
Brunner F, Andrew P, Wolkart G, Zechner R, Mayer B. Myocardial contractile function and heart rate in mice with myocyte-specific overexpression of endothelial nitric oxide synthase. Circulation. 2001;104(25):3097–102.
Janssens S, Pokreisz P, Schoonjans L, Pellens M, Vermeersch P, Tjwa M, et al. Cardiomyocyte-specific overexpression of nitric oxide synthase 3 improves left ventricular performance and reduces compensatory hypertrophy after myocardial infarction. Circ Res. 2004;94(9):1256–62. doi:10.1161/01.RES.0000126497.38281.23.
Ohashi Y, Kawashima S, Hirata K, Yamashita T, Ishida T, Inoue N, et al. Hypotension and reduced nitric oxide-elicited vasorelaxation in transgenic mice overexpressing endothelial nitric oxide synthase. J Clin Invest. 1998;102(12):2061–71. doi:10.1172/JCI4394.
van Haperen R, de Waard M, van Deel E, Mees B, Kutryk M, van Aken T, et al. Reduction of blood pressure, plasma cholesterol, and atherosclerosis by elevated endothelial nitric oxide. J Biol Chem. 2002;277(50):48803–7. doi:10.1074/jbc.M209477200.
Mungrue IN, Gros R, You X, Pirani A, Azad A, Csont T, et al. Cardiomyocyte overexpression of iNOS in mice results in peroxynitrite generation, heart block, and sudden death. J Clin Invest. 2002;109(6):735–43. doi:10.1172/JCI13265.
Heger J, Godecke A, Flogel U, Merx MW, Molojavyi A, Kuhn-Velten WN, et al. Cardiac-specific overexpression of inducible nitric oxide synthase does not result in severe cardiac dysfunction. Circ Res. 2002;90(1):93–9.
Takamura T, Kato I, Kimura N, Nakazawa T, Yonekura H, Takasawa S, et al. Transgenic mice overexpressing type 2 nitric-oxide synthase in pancreatic beta cells develop insulin-dependent diabetes without insulitis. J Biol Chem. 1998;273(5):2493–6.
Pfeifer A, Aszodi A, Seidler U, Ruth P, Hofmann F, Fassler R. Intestinal secretory defects and dwarfism in mice lacking cGMP-dependent protein kinase II. Science. 1996;274(5295):2082–6.
Pokreisz P, Pellens M, Van den Bergh A, Gillijns H, Bito V, Lenaers I, et al. Cardiomyocyte-specific overexpression of type 5 phosphodiesterase impairs postinfarction myocardial function and left ventricular remodeling. Circulation. 2007;116(16):45–6.
Disclosure
No potential conflicts of interest relevant to this article were reported.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Thoonen, R., Sips, P.Y., Bloch, K.D. et al. Pathophysiology of Hypertension in the Absence of Nitric Oxide/Cyclic GMP Signaling. Curr Hypertens Rep 15, 47–58 (2013). https://doi.org/10.1007/s11906-012-0320-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11906-012-0320-5