Abstract
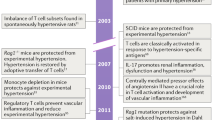
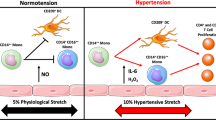
Inflammation plays an important role in the pathogenesis of hypertension. Innate and adaptive immune response may contribute to this process. The mechanisms implicating immune response in hypertension are still elusive. To date, the evidence originates in three major areas of data: cytokine production, central nervous system (CNS) stimulation, and kidney damage. The cytokine microenvironment can become proinflammatory and propagate low-grade inflammation, which may contribute to vascular injury and end-organ damage in hypertension. In addition, stimulation of the CNS by some stimuli (e.g., angiotensin II) causes mild hypertension that may modulate peripheral immune responses leading to aggravation of blood pressure elevation. The immune response can induce kidney injury and also interfere with sodium excretion, further contributing to elevation of blood pressure. The purpose of this review is to discuss recent data regarding the contribution of the different immune cell subsets and their response and mechanism of action in promoting hypertension and target-organ damage.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance•• Of major importance
Virdis A, Schiffrin EL. Vascular inflammation: a role in vascular disease in hypertension? Curr Opin Nephrol Hypertens. 2003;12(2):181–7.
Tian N, Moore RS, Braddy S, et al. Interactions between oxidative stress and inflammation in salt-sensitive hypertension. Am J Physiol Heart Circ Physiol. 2007;293(6):H3388–3395.
•• Harrison DG, Guzik TJ, Lob HE, et al. Inflammation, immunity, and hypertension. Hypertension 2011, 57(2):132–140. This excellent review of recent data focuses on the role of CNS stimuli in modulation of the immune system and hypertension.
Cruvinel W, Mesquita Jr D, Araújo JA, et al. Immune system - part I. Fundamentals of innate immunity with emphasis on molecular and cellular mechanisms of inflammatory response. Rev Bras Reumatol. 2010;50(4):434–61.
Miossec P, Korn T, Kuchroo VK. Interleukin-17 and type 17 helper T cells. N Engl J Med. 2009;361(9):888–98.
Sakaguchi S, Miyara M, Costantino CM, Hafler DA. FOXP3+ regulatory T cells in the human immune system. Nat Rev Immunol. 2010;10(7):490–500.
Okuda T, Grollman A. Passive transfer of autoimmune induced hypertension in the rat by lymph node cells. Tex Rep Biol Med. 1967;25(2):257–64.
Rodriguez-Iturbe B, Quiroz Y, Nava M, et al. Reduction of renal immune cell infiltration results in blood pressure control in genetically hypertensive rats. Am J Physiol Renal Physiol. 2002;282(2):F191–201.
Rodriguez-Iturbe B, Pons H, Quiroz Y, et al. Mycophenolate mofetil prevents salt-sensitive hypertension resulting from angiotensin II exposure. Kidney Int. 2001;59(6):2222–32.
Shao J, Nangaku M, Miyata T, et al. Imbalance of T-cell subsets in angiotensin II-infused hypertensive rats with kidney injury. Hypertension. 2003;42(1):31–8.
Guzik TJ, Hoch NE, Brown KA, et al. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med. 2007;204(10):2449–60.
• Crowley SD, Song YS, Lin EE, et al. Lymphocyte responses exacerbate angiotensin II-dependent hypertension. Am J Physiol Regul Integr Comp Physiol 2010;298(4):R1089-1097. Using mice that have severe combined immunodeficiency (SCID), which do not have T cells or B cells (in a manner similar to RAG-1 −/− mice), these investigators confirmed that T cells are essential for full development of angiotensin II–induced hypertension.
• Vinh A, Chen W, Blinder Y, et al. Inhibition and genetic ablation of the B7/CD28 T-cell costimulation axis prevents experimental hypertension. Circulation 2010;122(24):2529–2537. The authors demonstrated that costimulation of T cells is critical for the development of blood pressure elevation in response to angiotensin II.
• Madhur MS, Lob HE, McCann LA, et al. Interleukin 17 promotes angiotensin II-induced hypertension and vascular dysfunction. Hypertension 2010;55(2):500–507. Interleukin 17 deficiency was shown to lead to inability to sustain blood pressure elevation induced by angiotensin II.
•• Viel EC, Lemarie CA, Benkirane K, et al. Immune regulation and vascular inflammation in genetic hypertension. Am J Physiol Heart Circ Physiol 2010;298(3):H938-944. This is the first report suggesting the participation of Tregs in blood pressure elevation, oxidative stress, inflammation, and vascular remodeling in a model of genetic hypertension, the Dahl salt-sensitive rat.
• Kvakan H, Kleinewietfeld M, Qadri F, et al. Regulatory T cells ameliorate angiotensin II-induced cardiac damage. Circulation 2009;119(22):2904–2912. This study demonstrated that adoptive transfer of Tregs ameliorates cardiac damage from Ang II–induced hypertension.
•• Barhoumi T, Kasal DA, Li MW, et al. T regulatory lymphocytes prevent angiotensin II-induced hypertension and vascular injury. Hypertension 2011;57(3):469–476. This study demonstrated for the first time that adoptive transfer of Tregs has a blood pressure lowering effect. This beneficial effect was accompanied by changes in cytokine production and improvement of endothelial function.
Jurewicz M, McDermott DH, Sechler JM, et al. Human T and natural killer cells possess a functional renin-angiotensin system: further mechanisms of angiotensin II-induced inflammation. J Am Soc Nephrol. 2007;18(4):1093–102.
Hoch NE, Guzik TJ, Chen W, et al. Regulation of T-cell function by endogenously produced angiotensin II. Am J Physiol Regul Integr Comp Physiol. 2009;296(2):R208–216.
• Tinsley JH, South S, Chiasson VL, Mitchell BM. Interleukin-10 reduces inflammation, endothelial dysfunction, and blood pressure in hypertensive pregnant rats. Am J Physiol Regul Integr Comp Physiol 2010;298(3):R713-719. These investigators infused IL-10 in rats in a pregnancy-hypertension model (low-dose DOCA and 0.9% saline), and they were able to demonstrate that IL-10 has anti-inflammatory and antihypertensive activity.
• Kinsey GR, Sharma R, Huang L, et al. Regulatory T cells suppress innate immunity in kidney ischemia-reperfusion injury. J Am Soc Nephrol 2009;20(8):1744–1753. Using Foxp3-deficient Scurfy mice (deficient in Tregs) and IL-10 −/− mice versus wild-type mice as sources for adoptive transfer of T cells, these investigators demonstrated the protective effect of Tregs in ischemia-reperfusion injury. They also showed that this effect is mediated by IL-10.
Kinsey GR, Huang L, Vergis AL, et al. Regulatory T cells contribute to the protective effect of ischemic preconditioning in the kidney. Kidney Int. 2010;77(9):771–80.
Didion SP, Kinzenbaw DA, Schrader LI, et al. Endogenous interleukin-10 inhibits angiotensin II-induced vascular dysfunction. Hypertension. 2009;54(3):619–24.
Tran LT, MacLeod KM, McNeill JH. Chronic etanercept treatment prevents the development of hypertension in fructose-fed rats. Mol Cell Biochem. 2009;330(1–2):219–28.
Swanson MA, Lee WT, Sanders VM. IFN-gamma production by Th1 cells generated from naive CD4+ T cells exposed to norepinephrine. J Immunol. 2001;166(1):232–40.
Ganta CK, Lu N, Helwig BG, et al. Central angiotensin II-enhanced splenic cytokine gene expression is mediated by the sympathetic nervous system. Am J Physiol Heart Circ Physiol. 2005;289(4):H1683–1691.
• Lob HE, Marvar PJ, Guzik TJ, et al.: Induction of hypertension and peripheral inflammation by reduction of extracellular superoxide dismutase in the central nervous system. Hypertension 2010;55(2):277–283. In this and reference [28•], these investigators demonstrated that the CNS induces blood pressure elevation, probably through sympathetic activation, which somehow leads to immune activation and vascular inflammation.
• Marvar PJ, Thabet SR, Guzik TJ, et al. Central and peripheral mechanisms of T-lymphocyte activation and vascular inflammation produced by angiotensin II-induced hypertension. Circ Res 2010;107(2):263–270. In this and reference [27•], these investigators demonstrated that the CNS induces blood pressure elevation, probably through sympathetic activation, which somehow leads to immune activation and vascular inflammation.
Mattson DL, James L, Berdan EA, Meister CJ. Immune suppression attenuates hypertension and renal disease in the Dahl salt-sensitive rat. Hypertension. 2006;48(1):149–56.
• De Miguel C, Lund H, Mattson DL: High dietary protein exacerbates hypertension and renal damage in Dahl SS rats by increasing infiltrating immune cells in the kidney. Hypertension 2011;57(2):269–274. As in the previous report [29], the authors provide a mechanistic explanation for the importance of T lymphocytes in the pathogenesis of hypertension in a high-salt model. A high-protein diet on top of a high-salt diet increased lymphocyte infiltration in the kidney. These lymphocytes are an important source for kidney Ang II and oxidative stress.
• De Miguel C, Guo C, Lund H, et al.: Infiltrating T lymphocytes in the kidney increase oxidative stress and participate in the development of hypertension and renal disease. Am J Physiol Renal Physiol 2011;300(3):F734-742. In this and reference [32•], these authors report that T lymphocytes infiltrate the kidney and, through production of reactive oxygen species, lead to renal disease and blood pressure elevation.
• De Miguel C, Das S, Lund H, Mattson DL: T lymphocytes mediate hypertension and kidney damage in Dahl salt-sensitive rats. Am J Physiol Regul Integr Comp Physiol 2010;298(4):R1136-1142. In this and reference [31•], these authors report that T lymphocytes infiltrate the kidney and, through production of reactive oxygen species, lead to renal disease and blood pressure elevation.
Franco M, Martinez F, Quiroz Y, et al. Renal angiotensin II concentration and interstitial infiltration of immune cells are correlated with blood pressure levels in salt-sensitive hypertension. Am J Physiol Regul Integr Comp Physiol. 2007;293(1):R251–256.
Moore KJ, Tabas I. Macrophages in the pathogenesis of atherosclerosis. Cell. 2011;145(3):341–55.
De Ciuceis C, Amiri F, Brassard P, et al. Reduced vascular remodeling, endothelial dysfunction, and oxidative stress in resistance arteries of angiotensin II-infused macrophage colony-stimulating factor-deficient mice: evidence for a role in inflammation in angiotensin-induced vascular injury. Arterioscler Thromb Vasc Biol. 2005;25(10):2106–13.
Ko EA, Amiri F, Pandey NR, et al. Resistance artery remodeling in deoxycorticosterone acetate-salt hypertension is dependent on vascular inflammation: evidence from m-CSF-deficient mice. Am J Physiol Heart Circ Physiol. 2007;292(4):H1789–1795.
Rickard AJ, Morgan J, Tesch G, et al. Deletion of mineralocorticoid receptors from macrophages protects against deoxycorticosterone/salt-induced cardiac fibrosis and increased blood pressure. Hypertension. 2009;54(3):537–43.
•• Machnik A, Neuhofer W, Jantsch J, et al.: Macrophages regulate salt-dependent volume and blood pressure by a vascular endothelial growth factor-C-dependent buffering mechanism. Nat Med 2009;15(5):545–552. This intriguing report suggests a role for skin macrophages in the regulation of salt and water retention leading to elevated blood pressure.
•• Machnik A, Dahlmann A, Kopp C,et al.: Mononuclear phagocyte system depletion blocks interstitial tonicity-responsive enhancer binding protein/vascular endothelial growth factor C expression and induces salt-sensitive hypertension in rats. Hypertension 2010;55(3):755–761. This intriguing report suggests a role for skin macrophages in the regulation of salt and water retention leading to elevated blood pressure.
Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8(12):958–69.
Acknowledgments
Work from the authors’ laboratory was financed by the Canadian Institutes of Health Research (CIHR) grants 37917, 82790, and 102606, a Canada Research Chair on Hypertension and Vascular Research from CIHR/Government of Canada, and the Canada Fund for Innovation (all to ELS).
Disclosure
Conflicts of Interest: A. Leibowitz: none; E.L. Schiffrin: Advisory Board membership and honoraria from Bristol-Myers Squibb, Daiichi-Sankyo, Novartis, Takeda; research grant from Novartis; fees for educational presentations or service on speakers’ bureau from Abbott, Bristol-Myers Squibb, Daiichi-Sankyo, Novartis, and Takeda (none related to the present manuscript).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Leibowitz, A., Schiffrin, E.L. Immune Mechanisms in Hypertension. Curr Hypertens Rep 13, 465–472 (2011). https://doi.org/10.1007/s11906-011-0224-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11906-011-0224-9