Abstract
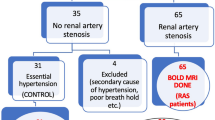
Establishing whether large vessel occlusive disease threatens tissue oxygenation and viability in the post-stenotic kidney is difficult for clinicians. Development of blood oxygen level–dependent (BOLD) MRI methods can allow functional evaluation of regional differences in deoxyhemoglobin levels within the kidney without requiring contrast. The complex renal circulation normally provides a gradient of oxygenation from a highly vascular cortex to much reduced levels in the deep sections of medulla, dependent upon adjustments in renal afferent arterioles, oxygen consumption related to solute transport, and arteriovenous shunting related to the juxtaposition of descending and ascending vasa recta. Studies with BOLD imaging have identified adaptation to substantial reductions in renal blood flow, volume, and glomerular filtration rate in post-stenotic kidneys that preserves medullary and cortical oxygenation during medical therapy. However, extreme vascular compromise overwhelms these adaptive changes and leads to cortical hypoxia and microvascular injury.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
• de Mast Q, Beutler JJ. The prevalence of atherosclerotic renal artery stenosis in risk groups: a systematic literature review. J Hypertens 2009; 27:1333–1340. This useful assembly of previous publications identifies ARAS as part of imaging for other indications. Of patients with suspected “renovascular hypertension,” 14.1% were positive for ARAS. Use of a low threshold (50% occlusion) and single publications regarding end-stage renal disease and congestive heart failure have overstated the risk.
Hackam DG, Duong-Hua ML, Mamdani M, Li P, Tobe SW, Spence JD, et al. Angiotensin inhibition in renovascular disease: a population-based cohort study. Am Heart J. 2008;156:549–55.
•• The ASTRAL Investigators. Revascularization versus medical therapy for renal-artery stenosis. N Engl J Med 2009; 361:1953–1962. This important but limited study selected 806 subjects by “uncertainty” as to whether they would benefit from revascularization as compared with medical therapy alone. Remarkably stable levels of kidney function overall did not differ between groups. These data emphasize the success and stability of medical therapy alone for many patients with ARAS for several years. More than 40% of the patients were in the category of 50% to 70% stenosis, which likely diluted the power of this trial.
Bax L, Woittiez AJ, Kouwenberg HJ, Mali PTM, Buskens E, Beek FJA, et al. Stent placement in patients with atherosclerotic renal artery stenosis and impaired renal function. Ann Intern Med. 2009;150:840–8.
Textor SC, McKusick MA, Misra S, Glockner J. Timing and selection for renal revascularization in an era of negative trials: what to do? Prog Cardiovasc Dis. 2009;52:220–8.
Heyman SN, Khamaisi M, Rosen S, Rosenberger C. Renal parenchymal hypoxia, hypoxia response and the progression of chronic kidney disease. Am J Nephrol. 2008;28:998–1006.
•• O’Connor PM, Evans RG. Structural antioxidant defense mechanisms in the mammalian and nonmammalian kidney: different solutions to the same problem? Am J Physiol Regul Integr Comp Physiol 2010; 299:R723-R727. This is a thoughtful reference regarding complex intrarenal mechanisms to protect oxygenation at both levels—avoiding hyperoxia and hypoxia by using unique characteristics within the kidney.
• Evans RG, Gardiner BS, Smith DW, O’Connor PM. Intrarenal oxygenation: unique challenges and the biophysical basis of homeostasis. Am J Physiol Renal Physiol 2008; 295:F1259-F1270. This important review of pathways of oxygenation regulation within the kidney supports the multiple roles of blood flow, oxygen consumption, and arteriovenous shunting.
Textor SC, Lerman L. Renovascular hypertension and ischemic nephropathy: state of the art. Am J Hypertens. 2010;23:1159–69.
Evans RG, Eppel GA, Michaels S, Burke SL, Nematbakhsh M, Head GA, et al. Multiple mechanisms act to maintain kidney oxygenation during renal ischemia in anesthetized rabbits. Am J Physiol Renal Physiol. 2010;298:F1235–43.
O’Connor PM, Kett MM, Warwick RP, Evans RG. Renal medullary tissue oxygenation is dependent on both cortical and medullary blood flow. Am J Physiol Renal Physiol. 2006;290:F688–94.
Sadowski J, Badzynska B. Specific features and roles of renal circulation: angiotensin II revisited. J Physiol and Pharmacol. 2006;57 Suppl 11:169–78.
•• Warner L, Glockner JF, Woollard J, Textor SC, Romero JC, Lerman LO. Determinations of renal cortical and medullary oxygenation using blood oxygen level-dependent magnetic resonance imaging and selective diuretics. Invest Radiol 2011; 46:41–47. This study of changes in tissue oxygenation after blockade of solute transport with furosemide, acetazolamide, or both emphasizes the role of oxygen consumption as a key determinant of medullary oxygenation in the normal kidney.
Gomez SI, Warner L, Haas JA, Bolterman RJ, Textor SC, Lerman LO, et al. Increased hypoxia and reduced renal tubular response to furosemide detected by BOLD magnetic resonance imaging in swine renovascular hypertension. Am J Physiol Renal Physiol. 2009;297:F981–6.
Gloviczki ML, Glockner J, Gomez SI, Romero JC, Lerman LO, McKusick M, et al. Comparison of 1.5 and 3 T BOLD MR to study oxygenation of kidney cortex and medulla in human renovascular disease. Invest Radiol. 2009;44:566–71.
•• Li L, Lin J, Santos EA, Dunkle E, Pierchala L, Prasad P. Effect of nitric oxide synthase inhibition on intrarenal oxygenation as evaluated by blood oxygenation level-dependent magnetic resonance imaging. Invest Radiol 2009; 44:67–73. This important study demonstrates that a rise in R2* in medullary regions may be induced by alterations in medullary blood flow, in addition to changes in oxygen consumption.
Palm F, Connors SG, Mendonca M, Welch WJ, Wilcox CS. Angiotensin II type 2 receptors and nitric oxide sustain oxygenation in the clipped kidney of early Goldblatt hypertensive rats. Hypertension. 2008;51:1–7.
Pedersen M, Dissing TH, Morkenborg J, Stodkilde-Jorgensen H, Hansen LH, Pedersen LB, et al. Validation of quantitative BOLD MRI measurements in the kidney: application to unilateral ureteral obstruction. Kidney Int. 2005;67:2305–12.
Warner L, Gomez SI, Bolterman R, Haas JA, Bentley MD, Lerman LO, et al. Regional decrease in renal oxygenation during graded acute renal arterial stenosis: a case for renal ischemia. Am J Physiol Regul Integr Comp Physiol. 2009;296:R67–71.
Juillard L, Lerman LO, Kruger DG, Haas JA, Rucker BC, Polzin JA, et al. Blood oxygen level-dependent measurement of acute intra-renal ischemia. Kidney Int. 2004;65:944–50.
Evans RG, Leong CL, Anderson WP, O’Connor PM. Don’t be so BOLD: potential limitations in the use of BOLD MRI for studies of renal oxygenation. Kidney Int. 2007;71:1327–8.
Hofmann L, Simon-Zoula S, Nowak A, Giger A, Vock P, Boesch C, et al. BOLD-MRI for the assessment of renal oxygenation in humans: acute effect of nephrotoxic xenobiotics. Kidney Int. 2006;70:144–50.
Djamali A, Sadowski EA, Muehrer RJ, Reese S, Smavatkul C, Vidyasagar A, et al. BOLD-MRI assessment of intrarenal oxygenation and oxidative stress in patients with chronic kidney allograft dysfunction. Am J Physiol Renal Physiol. 2007;292:F513–22.
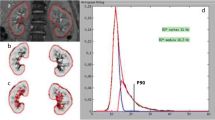
Textor SC, Glockner JF, Lerman LO, Misra S, McKusick MA, Riederer SJ, et al. The use of magnetic resonance to evaluate tissue oxygenation in renal artery stenosis. J Am Soc Nephrol. 2008;19:780–8.
•• Gloviczki ML, Glockner JF, Lerman LO, McKusick MA, Misra S, Grande JP, et al. Preserved oxygenation despite reduced blood flow in poststenotic kidneys in human atherosclerotic renal artery stenosis. Hypertension 2010; 55:961–966. Data from carefully conducted studies in humans with ARAS apply BOLD MRI to demonstrate well-preserved cortical and medullary oxygen levels despite substantial reduction in blood flow and kidney size for most individuals. These observations partly explain the stability of kidney function in patients treated medically in the ASTRAL trial.
Pedersen M, Lausten C, Perot V, Basseau F, Moonen C, Grenier N. Renal hemodynamics and oxygenation in transient renal artery occluded rats evaluated with iron-oxide particles and oxygenation-sensitive imaging. Z Med Phys. 2010;20:134–42.
•• Pruijm M, Hofmann L, Maillard M, Tremblay S, Glatz N, Wuerzner G, et al. Effect of sodium loading/depletion on renal oxygenation and young normotensive and hypertensive men. Hypertension 2010; 55:1116–1122. This important study used repeated BOLD MRI studies to identify effects of changing sodium intake. The results demonstrated a major fall in R2* in medullary segments during low sodium intake, consistent with reduced medullary oxygen consumption related to reduced sodium transport.
Lerman LO, Textor SC, Grande JP. The mechanisms of tissue injury in renal artery stenosis: ischemia and beyond. Prog Cardiovasc Dis. 2009;52:196–203.
• Cheng J, Zhou W, Warner GM, Knudsen BE, Garovic VD, Gray CE, et al. Temporal analysis of signaling pathways activated in a murine model of two-kidney, one-clip hypertension. Am J Physiol Renal Physiol 2009; 297:F1055-F1068. This study used a murine model of renal artery stenosis to demonstrate a sequential increase of p-ERK pathways, proliferating cell markers, and transforming growth factor-beta (TGF-β) in poststenotic kidneys preceding the development of interstitial fibrosis and irreversible tissue injury.
Rognant N, Guebre-Egziabher F, Bachetta J, Janier M, Hiba B, Langlois JB, et al. Evolution of renal oxygen content measured by BOLD MRI downstream in a chronic renal artery stenosis. Nephrol Dial Transplant. 2011;26:1205–10.
• Cheung CM, Chrysochou C, Shurrab AE, Buckley DL, Cowie A, Kalra PA. Effects of renal volume and single-kidney glomerular filtration rate on renal functional outcome in atherosclerotic renal artery stenosis. Nephrol Dial Transplant 2010; 25:1133–1140. This interesting study combines measurement of preserved kidney volume (by MR) and single-kidney GFR (by radioisotope scanning). These authors demonstrate greater likelihood of recovering GFR after revascularizing the kidney when a discrepancy is observed between preserved volume and reduced GFR, suggesting “hibernating” kidney function in renal artery stenosis.
Gloviczki ML, Keddis MT, McKusick MA, Misra S, Grande JP, Lerman LO, et al. Transvenous biopsies of kidneys beyond renal artery stenosis demonstrate fibrosis correlated to reduced single kidney blood flow, but not urinary or blood TGF-beta levels [abstract]. American Society of Nephrology 2010; abstract F-P01703
Zhu XY, Chade AR, Rodriguez-Porcel M, Bentley MD, Ritman EL, Lerman A, et al. Cortical microvascular remodeling in the stenotic kidney: role of increased oxidative stress. Arterioscler Thromb Vasc Biol. 2004;24:1854–9.
•• Favreau F, Zhu XY, Krier JD, Lin J, Warner L, Textor SC, Lerman LO. Revascularization of swine renal artery stenosis improves renal function but not the changes in vascular structure. Kidney Int 2010; 78:1110–1118. This important paper identifies a failure of microvascular structures and angiogenic cytokines to normalize despite restoration of patent arteries in experimental renal artery stenosis.
Eirin A, Zhu XY, Urbieta-Caceres VH, Grande JP, Lerman A, Textor SC, et al. Persistent kidney dysfunction in swine renal artery stenosis correlates with outer cortical microvascular remodeling. Am J Physiol Renal Physiol. 2011;300:F1394–1401.
•• Chade AR, Zhu X, Lavi R, Krier JD, Pislaru S, Simari RD, et al. Endothelial progenitor cells restore renal function in chronic experimental renovascular disease. Circulation 2009; 119:547–557. This important experimental study demonstrates the potential for circulating pluripotent progenitor cells to shape the recovery of viable blood vessels, improve filtration, and restore tubular function in kidneys that are undergoing vascular rarefaction beyond a renal artery stenosis. These data provide a rationale for considering stem cells as an adjunctive maneuver in recovering kidney structure in ischemic renal disease.
Welch WJ, Blau J, Xie H, Chabrashvili T, Wilcox CS. Angiotensin-induced defects in renal oxygenation: role of oxidative stress. Am J Physiol Heart Circ Physiol. 2005;288:H22–8.
Lubbers DW, Baumgartl H. Heterogeneities and profiles of oxygen tension in brain and kidney as examples of PO2 distribution in the living tissue. Kidney Int. 1997;51:372–80.
Prasad P, Li LP, Halter SH, Cabray J, Ye M, Battle D. Evaluation of renal hypoxia in diabetic mice by BOLD MRI. Invest Radiol. 2010;45:819–22.
Li L, Vu AT, Li BSY, Dunkle E, Prasad PV. Evaluation of intrarenal oxygenation by BOLD MRI at 3.0 T. J Magn Reson Imaging. 2004;20:901–4.
Tumkur SM, Vu AT, Li LP, Pierchala L, Prasad PV. Evaluation of intra-renal oxygenation during water diuresis: a time-resolved study using BOLD MRI. Kidney Int. 2006;70:139–43.
Acknowledgments
The projects described were supported by Award Number PO1HL85307 from the National Heart, Lung and Blood Institute and NIH/NCRR CTSA Grant Number UL1 RR024150. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, and Blood Institute or the National Institutes of Health.
Disclosure
No potential conflicts of interest relevant to this article were reported.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gloviczki, M.L., Lerman, L.O. & Textor, S.C. Blood Oxygen Level–Dependent (BOLD) MRI in Renovascular Hypertension. Curr Hypertens Rep 13, 370–377 (2011). https://doi.org/10.1007/s11906-011-0218-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11906-011-0218-7