Abstract
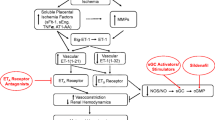
Reduced uterine perfusion pressure during pregnancy is an important initiating event in preeclampsia. Inflammatory cytokines are thought to link placental ischemia with cardiovascular and renal dysfunction. Supporting a role for cytokines are findings of elevated tumor necrosis factor (TNF)-α and interleukin (IL)-6 plasma levels in preeclamptic women. Blood pressure regulatory systems (eg, renin-angiotensin system [RAS] and sympathetic nervous system) interact with proinflammatory cytokines, which affect angiogenic and endothelium-derived factors regulating endothelial function. Chronic reductions in placental perfusion in pregnant rats are associated with enhanced TNF-α and IL-6 production. Chronic infusion of TNF-α or IL-6 into normal pregnant rats significantly increases arterial pressure and impairs renal hemodynamics. TNF-α activates the endothelin system in placental, renal, and vascular tissues, and IL-6 stimulates the RAS. These findings suggest that inflammatory cytokines elevate blood pressure during pregnancy by activating multiple neurohumoral and endothelial factors.
Similar content being viewed by others
References and Recommended Reading
Poston L: Endothelial dysfunction in preeclampsia. Pharmacol Rep 2006, 58:69–74.
McMaster MT, Ahou Y, Fisher SJ: Abnormal placentation and the syndrome of preeclampsia. Semin Nephrol 2004, 24:540–547.
Sharma A, Satyam A, Sharma JB: Leptin, IL-10, and inflammatory markers (TNF-alpha, IL-6 and IL-8) in preeclamptic, normotensive pregnant and healthy nonpregnant women. Am J Reprod Immunol 2007, 58:21–30.
Girardi G, Yarilin D, Thurman JM, et al.: Complement activation induces dysregulation of angiogenic factors and causes fetal rejection and growth restriction. J Exp Med 2006, 203:2165–2175.
Lee DL, Sturgis LC, Labazi H, et al.: Angiotensin II hypertension is attenuated in interleukin-6 knockout mice. Am J Physiol Heart Circ Physiol 2006, 288:H935–H940.
Gadonski G, LaMarca BB, Sullivan E, et al.: Hypertension produced by reductions in uterine perfusion in the pregnant rat: role of interleukin-6. Hypertension 2006, 48:711–716.
LaMarca BB, Cockrell K, Sullivan E, et al.: Role of endothelin in mediating tumor necrosis factor-induced hypertension in pregnant rats. Hypertension 2005, 46:82–86.
Maruotti N, Canatore FP, Crivellato E, et al.: Angiogenesis in rheumatoid arthritis. Histol Histopathol 2006, 21:557–566.
Granger JP, LaMarca BBD, Cockrell K, et al.: Reduced uterine perfusion pressure (RUPP) model for studying cardiovascular-renal dysfunction in response to placental ischemia. Methods Mol Med 2006, 122:383–392.
LaMarca BBD, Bennett WA, Alexander BT, et al.: Hypertension produced by reductions in uterine perfusion in the pregnant rat: role of tumor necrosis factor alpha. Hypertension 2005, 46:1022–10225.
Roberts L, LaMarca B, Fournier L, et al.: Enhanced endothelin synthesis by endothelial cells exposed to sera from pregnant rats with decreased uterine perfusion. Hypertension 2006, 47:615–618.
Bdolah Y, Sukhatme VP, Karumanchi SA: Angiogenic imbalance in the pathophysiology of preeclampsia: newer insights. Semin Nephrol 2004, 24:548–556.
Shibata E, Rajakumar A, Powers RW, et al.: Soluble fms-like tyrosine kinase 1 is increased in preeclampsia but not in normotensive pregnancies with small-for-gestational-age neonates: relationship to circulating placental growth factor. J Clin Endocrinol Metab 2005, 90:4895–4903.
Wolf M, Shah A, Lam C, et al.: Circulating levels of the antiangiogenic marker sFLT-1 are increased in first versus second pregnancies. Am J Obstet Gynecol 2005, 193:16–22.
Maynard SE, Venkatesha S, Thadhani R, et al.: Soluble Fms-like tyrosine kinase 1 and endothelial dysfunction in the pathogenesis of preeclampsia. Pediatr Res 2005, 57:1R–7R.
Karumanchi SA, Bdolah Y: Hypoxia and sFlt-1 in preeclampsia: the “chicken-and-egg” question. Endocrinology 2004, 145:4835–4837.
Karumanchi SA, Maynard SE, Stillman IE, et al.: Preeclampsia: a renal perspective. Kidney Int 2005, 67:2101–2113.
Shimizu A, Masuda Y, Mori T, et al.: Vascular endothelial growth factor 165 resolves glomerular inflammation and accelerates glomerular capillary repair in rat anti-glomerular basement membrane glomerulonephritis. J Am Soc Nephrol 2004, 15:2655–2665.
Miyamoto K, Kitamoto Y, Tokunaga H, et al.: Protective effect of vascular endothelial growth factor/vascular permeability factor 165 and 121 on glomerular endothelial cell injury in the rat. Lab Invest 2004, 84:1126–1136.
Hara A, Wada T, Furuichi K, et al.: Blockade of VEGF accelerates proteinuria, via decrease in nephrin expression in rat crescentic glomerulonephritis. Kidney Int 2006, 69:1986–1995.
Bujold E, Romero R, Chaiworapongsa T, et al.: Evidence supporting that the excess of the sVEGFR-1 concentration in maternal plasma in preeclampsia has a uterine origin. J Matern Fetal Neonatal Med 2005, 18:9–16.
Rajakumar A, Michael HM, Rajakumar PA, et al.: Extraplacental expression of vascular endothelial growth factor receptor-1, (Flt-1) and soluble Flt-1 (sFlt-1), by peripheral blood mononuclear cells (PBMCs) in normotensive and preeclamptic pregnant women. Placenta 2005, 26:563–573.
Krysiak O, Bretschneider A, Zhong E, et al.: Soluble vascular endothelial growth factor receptor-1 (sFLT-1) mediates downregulation of FLT-1 and prevents activated neutrophils from women with preeclampsia from additional migration by VEGF. Circ Res 2005, 97:1253–1261.
Nadar SK, Karalis I, Al Yemeni E, et al.: Plasma markers of angiogenesis in pregnancy induced hypertension. Thromb Haemost 2005, 94:1071–1076.
Koyama S, Kimura T, Ogita K, et al.: Transient local overexpression of human vascular endothelial growth factor (VEGF) in mouse feto-maternal interface during midterm pregnancy lowers systemic maternal blood pressure. Hormone and Metabolic Research 2006, 38:619–624.
Thadhani RI, Johnson RJ, Karumanchi SA: Hypertension during pregnancy: a disorder begging for pathophysiological support. Hypertension 2005, 46:1250–1251.
Thadhani R, Mutter WP, Wolf M, et al.: First trimester placental growth factor and soluble fms-like tyrosine kinase 1 and risk for preeclampsia. J Clin Endocrinol Metab 2004, 89:770–775.
Levine RJ, Maynard SE, Qian C, et al.: Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med 2004, 350:672–683.
Nevo O, Soleymanlou N, Wu Y, et al.: Increased expression of sFlt-1 in in vivo and in vitro models of human placental hypoxia is mediated by HIF-1. Am J Physiol Regul Integr Comp Physiol 2006, 291:R1085–R1093.
Eubank TD, Roberts R, Galloway M, et al.: GM-CSF induces expression of soluble VEGF receptor-1 from human monocytes and inhibits angiogenesis in mice. Immunity 2004, 21:831–842.
Walther T, Wallukat G, Alexander J, et al.: Angiotensin II type 1 receptor agonistic antibodies reflect fundamental alterations in the uteroplacental vasculature. Hypertension 2005, 46:1275–1279.
Hubel CA, Wallukat G, Wolf M, et al.: Agonistic angiotensin II type 1 receptor autoantibodies in postpartum women with a history of preeclampsia. Hypertension 2007, 49:612–617.
Zhou CC, Ahmad S, Mi T, et al.: Angiotensin II induces soluble fms-like tyrosine kinase-1 release via calcineurin signaling pathway in pregnancy. Circ Res 2007, 100:88–95.
Stennett AK, Khalil RA: Neurovascular mechanisms of hypertension in pregnancy. Curr Neurovasc Res 2006, 3:131–148.
Zhang ZH, Wei SG, Francis J, et al.: Cardiovascular and renal sympathetic activation by blood-borne TNF-alpha in rat: the role of central prostaglandins. Am J Physiol Regul Integr Comp Physiol 2003, 284:R916–R927.
Ye S, Mozayeni P, Gamburd M, et al.: Interleukin-1beta and neurogenic control of blood pressure in normal rats and rats with chronic renal failure. Am J Physiol Heart Circ Physiol 2000; 279:H2786–H2796.
Campese VM, Ye S, Zhong H: Downregulation of neuronal nitric oxide synthase and interleukin-1beta mediates angiotensin II-dependent stimulation of sympathetic nerve activity. Hypertension 2002, 39:519–524.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
LaMarca, B.D., Ryan, M.J., Gilbert, J.S. et al. Inflammatory cytokines in the pathophysiology of hypertension during preeclampsia. Current Science Inc 9, 480–485 (2007). https://doi.org/10.1007/s11906-007-0088-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11906-007-0088-1