Abstract
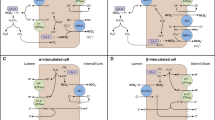
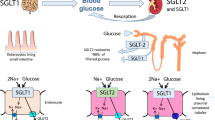
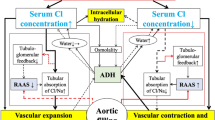
The post-macula densa segments of the renal tubule—that is, the distal convoluted tubule, connecting tubule, and collecting duct—play a central role in determining final urine sodium excretion. The major regulated sodium transporters and channels in these cell types include the thiazide-sensitive (Na-Cl) cotransporter (NCC), the epithelial sodium channel (ENaC), and Na-K-ATPase. Furthermore, although not involved in sodium reabsorption, the anion exchanger, pendrin, and the basolateral bumetanide-sensitive Na-K-2Cl cotransporter (NKCC1 or BSC2) have roles in blood-volume maintenance. Mutations in several of these major sodium transporters, channel subunits, and their regulatory proteins have been linked to human diseases such as Liddle’s syndrome, Gitelman’s syndrome, and Gordon’s syndrome, emphasizing the need for appropriate regulation of sodium at these sites for maintenance of sodium balance and normotension.
Similar content being viewed by others
References and Recommended Reading
Bianchi G, Fox U, Di Francesco GF, et al.: Blood pressure changes produced by kidney cross-transplantation between spontaneously hypertensive rats and normotensive rats. Clin Sci Mol Med 1974, 47:435–448.
Vander AJ: Basic renal processes for sodium, chloride, and water. In Renal Physiology, edn 5. New York: McGraw-Hill; 1995:89–115.
Lifton RP: Molecular genetics of human blood pressure variation. Science 1996, 272:676–680.
Bachmann S, Kriz W: Histology, cytology, ultrastructure. Nephron and collecting duct structure in the kidney rat. In Urinary System. Edited by Jones TC, Hard GC, Mohr U. Heidelberg: Springer; 1998.
Lingrel JB: Na,K-ATPase: isoform structure, function, and expression. J Bioenerg Biomembr 1992, 24:263–270.
El Mernissi G, Doucet A: Quantitation of [3H]ouabain binding and turnover of Na-K-ATPase along the rabbit nephron. Am J Physiol 1984, 247:F158-F167.
Velazquez H, Bartiss A, Bernstein P, et al.: Adrenal steroids stimulate thiazide-sensitive NaCl transport by rat renal distal tubules. Am J Physiol 1996, 270:F211-F219.
Ellison DH, Velazquez H, Wright FS: Thiazide-sensitive sodium chloride cotransport in early distal tubule. Am J Physiol 1987, 253:F546-F554.
Plotkin MD, Kaplan MR, Verlander JW, et al.: Localization of the thiazide sensitive Na-Cl cotransporter, rTSC1 in the rat kidney. Kidney Int 1996, 50:174–183.
Firsov D, Gautschi I, Merillat AM, et al.: The heterotetrameric architecture of the epithelial sodium channel (ENaC). EMBO J 1998, 17:344–352.
Snyder PM, Cheng C, Prince LS, et al.: Electrophysiological and biochemical evidence that DEG/ENaC cation channels are composed of nine subunits. J Biol Chem 1998, 273:681–684.
Duc C, Farman N, Canessa CM, et al.: Cell-specific expression of epithelial sodium channel alpha, beta, and gamma subunits in aldosterone-responsive epithelia from the rat: localization by in situ hybridization and immunocytochemistry. J Cell Biol 1994, 127:1907–1921.
Everett LA, Green ED: A family of mammalian anion transporters and their involvement in human genetic diseases. Hum Mol Genet 1999, 8:1883–1891.
Kim YH, Kwon TH, Frische S, et al.: Immunocytochemical localization of pendrin in intercalated cell subtypes in rat and mouse kidney. Am J Physiol Renal Physiol 2002, 283:F744-F754.
Verlander JW, Hassell KA, Royaux IE, et al.: Deoxycorticosterone upregulates PDS (Slc26a4) in mouse kidney: role of pendrin in mineralocorticoid-induced hypertension. Hypertension 2003, 42:356–362. This article is important, as it shows the upregulation of pendrin with aldosterone analogues, which could be critical in the pathogenesis of mineralocorticoid-induced hypertension and metabolic alkalosis.
Royaux IE, Wall SM, Karniski LP, et al.: Pendrin, encoded by the Pendred syndrome gene, resides in the apical region of renal intercalated cells and mediates bicarbonate secretion. Proc Natl Acad Sci U S A 2001, 98:4221–4226.
Wall SM, Kim YH, Stanley L, et al.: NaCl restriction upregulates renal Slc26a4 through subcellular redistribution: role in Cl-conservation. Hypertension 2004, 44:982–987.
Ginns SM, Knepper MA, Ecelbarger CA, et al.: Immunolocalization of the secretory isoform of Na-K-Cl cotransporter in rat renal intercalated cells. J Am Soc Nephrol 1996, 7:2533–2542.
Kaplan MR, Mount DB, Delpire E: Molecular mechanisms of NaCl cotransport. Annu Rev Physiol 1996, 58:649–668.
Meyer JW, Flagella M, Sutliff RL, et al.: Decreased blood pressure and vascular smooth muscle tone in mice lacking basolateral Na(+)-K(+)-2Cl(-) cotransporter. Am J Physiol Heart Circ Physiol 2002, 283:H1846-H1855.
Wall SM, Knepper MA, Hassell KA, et al.: Hypotension in NKCC1 null mice: role of the kidneys. Am J Physiol Renal Physiol 2005, Epub ahead of print. This article is important, as it shows that the action of aldosterone and vasopressin is altered within kidneys of NKCC1 null mice, which likely contributes to their hypotension.
Lebeau C, Hanaoka K, Moore-Hoon ML, et al.: Basolateral chloride transporters in autosomal dominant polycystic kidney disease. Pflugers Arch 2002, 444:722–731.
Cuffe JE, Howard DP, Bertog M, et al.: Basolateral adrenoceptor activation mediates noradrenaline-induced Cl-secretion in M-1 mouse cortical collecting duct cells. Pflugers Arch 2002, 445:381–389.
Bostanjoglo M, Reeves WB, Reilly RF, et al.: 11Betahydroxysteroid dehydrogenase, mineralocorticoid receptor, and thiazide-sensitive Na-Cl cotransporter expression by distal tubules. J Am Soc Nephrol 1998, 9:1347–1358.
Vinciguerra M, Mordasini D, Vandewalle A, et al.: Hormonal and nonhormonal mechanisms of regulation of the NA,K-pump in collecting duct principal cells. Semin Nephrol 2005, 25:312–321.
Masilamani S, Kim GH, Mitchell C, et al.: Aldosterone-mediated regulation of ENaC alpha, beta, and gamma subunit proteins in rat kidney. J Clin Invest 1999, 104:R19-R23.
Kim GH, Masilamani S, Turner R, et al.: The thiazidesensitive Na-Cl cotransporter is an aldosterone-induced protein. Proc Natl Acad Sci U S A 1998, 95:14552–14557.
Nielsen J, Kwon TH, Masilamani S, et al.: Sodium transporter abundance profiling in kidney: effect of spironolactone. Am J Physiol Renal Physiol 2002, 283:F923-F933.
Masilamani S, Wang X, Kim GH, et al.: Time course of renal Na-K-ATPase, NHE3, NKCC2, NCC, and ENaC abundance changes with dietary NaCl restriction. Am J Physiol Renal Physiol 2002, 283:F648-F657.
Wang XY, Masilamani S, Nielsen J, et al.: The renal thiazide-sensitive Na-Cl cotransporter as mediator of the aldosterone-escape phenomenon. J Clin Invest 2001, 108:215–222.
Turban S, Wang XY, Knepper MA: Regulation of NHE3, NKCC2, and NCC abundance in kidney during aldosterone escape phenomenon: role of NO. Am J Physiol Renal Physiol 2003, 285:F843-F851.
Beutler KT, Masilamani S, Turban S, et al.: Long-term regulation of ENaC expression in kidney by angiotensin II. Hypertension 2003, 41:1143–1150.
Brooks HL, Allred AJ, Beutler KT, et al.: Targeted proteomic profiling of renal Na(+) transporter and channel abundances in angiotensin II type 1a receptor knockout mice. Hypertension 2002, 39:470–473.
Ecelbarger CA, Kim GH, Wade JB, et al.: Regulation of the abundance of renal sodium transporters and channels by vasopressin. Exp Neurol 2001, 171:227–234.
Ecelbarger CA, Kim GH, Terris J, et al.: Vasopressin-mediated regulation of epithelial sodium channel abundance in rat kidney. Am J Physiol Renal Physiol 2000, 279:F46-F53.
Djelidi S, Fay M, Cluzeaud F, et al.: Transcriptional regulation of sodium transport by vasopressin in renal cells. J Biol Chem 1997, 272:32919–32924.
Bankir L, Fernandes S, Bardoux P, et al.: Vasopressin-V2 receptor stimulation reduces sodium excretion in healthy humans. J Am Soc Nephrol 2005, 16:1920–1928.
Song J, Hu X, Khan O, et al.: Increased blood pressure, aldosterone activity, and regional differences in renal ENaC protein during vasopressin escape. Am J Physiol Renal Physiol 2004, 287:F1076-F1083.
Ecelbarger CA, Knepper MA, Verbalis JG: Increased abundance of distal sodium transporters in rat kidney during vasopressin escape. J Am Soc Nephrol 2001, 12:207–217.
Atchley D, Loeb RF, Richards DW, et al.: On diabetic acidosis. J Clin Invest 1933, 12:297–326.
DeFronzo RA, Cooke CR, Andres R, et al.: The effect of insulin on renal handling of sodium, potassium, calcium, and phosphate in man. J Clin Invest 1975, 55:845–855.
DeFronzo RA, Goldberg M, Agus ZS: The effects of glucose and insulin on renal electrolyte transport. J Clin Invest 1976, 58:83–90.
Chibalin AV, Kovalenko MV, Ryder JW, et al.: Insulin- and glucose-induced phosphorylation of the Na(+),K(+)-adenosine triphosphatase alpha-subunits in rat skeletal muscle. Endocrinology 2001, 142:3474–3482.
Feraille E, Rousselot M, Rajerison R, et al.: Effect of insulin on Na+,K(+)-ATPase in rat collecting duct. J Physiol 1995, 488:171–180.
Bickel CA, Verbalis JG, Knepper MA, et al.: Increased renal Na-K-ATPase, NCC, and beta-ENaC abundance in obese Zucker rats. Am J Physiol Renal Physiol 2001, 281:F639-F648.
Khan O, Riazi S, Hu X, et al.: Regulation of the renal thiazide-sensitive Na-Cl cotransporter, blood pressure, and natriuresis in obese Zucker rats treated with rosiglitazone. Am J Physiol Renal Physiol 2005, 289:F442-F450.
Verlander JW, Tran TM, Zhang L, et al.: Estradiol enhances thiazide-sensitive NaCl cotransporter density in the apical plasma membrane of the distal convoluted tubule in ovariectomized rats. J Clin Invest 1998, 101:1661–1669.
Chen Z, Vaughn DA, Fanestil DD: Influence of gender on renal thiazide diuretic receptor density and response. J Am Soc Nephrol 1994, 5:1112–1119.
Riazi S, Maric C, Ecelbarger C: 17-beta estradiol attenuates streptozotocin-induced diabetes and regulates the expression of renal sodium transporters. Kidney Int 2006, In press.
Carattino MD, Edinger RS, Grieser HJ, et al.: Epithelial sodium channel inhibition by AMP-activated protein kinase in oocytes and polarized renal epithelial cells. J Biol Chem 2005, 280:17608–17616.
Flores SY, Debonneville C, Staub O: The role of Nedd4/ Nedd4-like dependant ubiquitylation in epithelial transport processes. Pflugers Arch 2003, 446:334–338.
Grunder S, Firsov D, Chang SS, et al.: A mutation causing pseudohypoaldosteronism type 1 identifies a conserved glycine that is involved in the gating of the epithelial sodium channel. EMBO J 1997, 16:899–907.
Wong ZY, Stebbing M, Ellis JA, et al.: Genetic linkage of beta and gamma subunits of epithelial sodium channel to systolic blood pressure. Lancet 1999, 353:1222–1225.
Scheinman SJ, Guay-Woodford LM, Thakker RV, et al.: Genetic disorders of renal electrolyte transport. N Engl J Med 1999, 340:1177–1187. They have reviewed mutations affecting the ENaC and diureticsensitive sodium-transport proteins.
Mune T, White PC: Apparent mineralocorticoid excess: genotype is correlated with biochemical phenotype. Hypertension 1996, 27:1193–1199.
Lovati E, Ferrari P, Dick B, et al.: Molecular basis of human salt sensitivity: the role of the 11beta-hydroxysteroid dehydrogenase type 2. J Clin Endocrinol Metab 1999, 84:3745–3749.
Wilson FH, Disse-Nicodeme S, Choate KA, et al.: Human hypertension caused by mutations in WNK kinases. Science 2001, 293:1107–1112. The authors establish that mutations in two members of the WNK family of serine-threonine kinases cause PHAII. Their finding may offer new targets for the development of antihypertensive drugs.
Yang CL, Angell J, Mitchell R, et al.: WNK kinases regulate thiazide-sensitive Na-Cl cotransport. J Clin Invest 2003, 111:1039–1045.
Levy D, DeStefano AL, Larson MG, et al.: Evidence for a gene influencing blood pressure on chromosome 17. Genome scan linkage results for longitudinal blood pressure phenotypes in subjects from the framingham heart study. Hypertension 2000, 36:477–483.
DiPetrillo K, Coutermarsh B, Soucy N, et al.: Tumor necrosis factor induces sodium retention in diabetic rats through sequential effects on distal tubule cells. Kidney Int 2004, 65:1676–1683.
Song J, Knepper MA, Verbalis JG, et al.: Increased renal ENaC subunit and sodium transporter abundances in streptozotocin-induced type 1 diabetes. Am J Physiol Renal Physiol 2003, 285:F1125-F1137.
Dussol B, Moussi-Frances J, Morange S, et al.: A randomized trial of furosemide vs hydrochlorothiazide in patients with chronic renal failure and hypertension. Nephrol Dial Transplant 2005, 20:349–353.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ecelbarger, C.A., Tiwari, S. Sodium transporters in the distal nephron and disease implications. Current Science Inc 8, 158–165 (2006). https://doi.org/10.1007/s11906-006-0013-z
Issue Date:
DOI: https://doi.org/10.1007/s11906-006-0013-z