Abstract
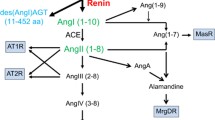
Angiotensinogen (AGT) can be schematically considered to consist of a combination of an angiotensin I (Ang I) function, located at the N-terminal end, and the presence of a serpin (serine protease inhibitor) structure at the opposite end. des(Ang I)AGT, which accounts for more than 97% of the molecule, apparently has no function. Several serpins (antithrombin, maspin, pigment epithelial-derived factor, and kallistatin) have been recently shown to exert an antiangiogenic activity, suggesting a common mechanism of endothelial cell proliferation and migration. AGT and its renin-cleaved product, des(Ang I)AGT, are also angiogenesis inhibitors, both in vitro and in vivo at concentrations within the range of those observed in plasma. This property most likely results from the structure analogy of AGT with serpins. The pathologic relevance of this new function is still not known, but AGT produced by glial cells may play a role in the stabilization of the blood-brain barrier. These new data must be considered in light of the overall action of the renin-angiotensin system in angiogenesis.
Similar content being viewed by others
References and Recommended Reading
Tewksbury D: Angiotensinogen. Biochemistry and molecular biology. In Hypertension. Edited by Laragh JH, Brenner BM. New York: Raven Press; 1990:1197–1216.
Jeunemaitre X, Soubrier F, Kotelevtsev Y, et al.: Molecular basis of human hypertension. Role of angiotensinogen. Cell 1992, 71:169–178.
Corvol P, Jeunemaitre X: Molecular genetics of human hypertension. Role of angiotensinogen. Endocrinol Rev 1997, 18:662–677.
Smithies O, Kim HS: Targeted gene duplication and disruption for analyzing quantitative genetic traits in mice. Proc Natl Acad Sci U S A 1994, 91:3612–3615.
Janciauskiene S: Conformational properties of serine proteinase inhibitors (serpins) confer multiple pathophysiological roles. Biochim Biophys Acta 2001, 1535:221–235. Comprehensive review of the different types of serpins and of their numerous roles, besides that of inhibiting serine esterase proteins.
Gaillard I, Clauser E, Corvol P: Structure of human angiotensinogen gene. DNA 1989, 8:87–99.
Stein PE, Tewkesbury DA, Carrell RW: Ovalbumin and angiotensinogen lack serpin S-R conformational change. Biochem J 1989, 262:103–107.
Mast AE, Enghild JJ, Pizzo SV, Salvesen G: Analysis of the plasma elimination kinetics and conformational stabilities of native, proteinase-complexed, and reactive site cleaved serpins: comparison of alpha 1-proteinase inhibitor, alpha 1-antichymotrypsin, antithrombin III, alpha 2-antiplasmin, angiotensinogen, and ovalbumin. Biochemistry 1999, 30:1723–1730.
Célérier J, Schmid G, Le Caer JP, et al.: Characterization of a human angiotensinogen cleaved in its reactive center loop by a proteolytic activity from Chinese hamster ovary cells. J Biol Chem 2000, 275:10648–10654. This study shows that AGT shares with other serpins the presence of a RCL and proposes a tertiary structure model of the protein based on that of á1-antitrypsin.
Becerra SP, Sagasti A, Spinella P, Notario V: Pigment epitheliumderived factor behaves like a noninhibitory serpin. Neurotrophic activity does not require the serpin reactive loop. J Biol Chem 1995, 270:25992–25999.
O’Reilly MS, Pirie-Shepherd S, Lane WS, Folkman J: Antiangiogenic activity of the cleaved conformation of the serpin antithrombin. Science 1999, 285:1926–1928. Surprising discovery of an antiangioenic effect of AT. This effect is independent from the inhibitory activity of AT, as cleaved AT is antiangiogenic.
Kisker O, Onizuka S, Banyard J, et al.: Generation of multiple angiogenesis inhibitors by human pancreatic cancer. Cancer Res 2001, 61:72938–7304.
Larsson H, Sjöblom T, Dixelius J, et al.: Antiangiogenic effects of latent antithrombin through perturbed cell-matrix interactions and apoptosis of endothelial cells. Cancer Res 2001, 60:6723–6729.
Zou Z, Anisowicz A, Hendrix MJ, et al.: Maspin, a serpin with tumor-suppressing activity in human mammary epithelial cells. Science 1994, 263:526–529.
Zhang M, Volpert O, Shi YH, Bouck N: Maspin is an angiogenesis inhibitor. Nat Med 2000, 6:196–199.
Blacque OE, Worrall DM: Evidence for a direct interaction between the tumor-suppressor serpin, maspin and types I and III collagen. J Biol Chem 2002, 277:10783–10788.
Dawson DW, Volpert OV, Gillis P, et al.: Pigment epitheliumderived factor: a potent inhibitor of angiogenesis. Science 1999, 285:245–248. Discovery of an antiangiogenic effect of PEDF. PEDF produced by retinal cells is modulated by oxygen concentration, suggesting that it plays a pathophysiologic role in ocular angiogenesis.
Spranger J, Osterhoff M, Reimann M, et al.: Loss of the antiangiogenic pigment epithelium-derived factor in patients with angiogenic eye disease. Diabetes 2001, 50:26141–2645.
Bouck N: PEDF: antiangiogenic guardian of ocular function. Trends Mol Med 2002, 8:330–334.
Stellmach V, Crawford SE, Zhou W, Bouck N: Prevention of ischemia-induced retinopathy by the natural ocular antiangiogenic agent pigment epithelium-derived factor. Proc Natl Acad Sci U S A 2001, 98:2593–2597.
Chao J, Stallone JN, Liang YM, et al.: Kallistatin is a potent new vasodilator. J Clin Invest 1997, 100:11–17.
Miao RQ, Agata J, Chao L, Chao J: Kallistatin is a new inhibitor of angiogenenis and tumor growth. Blood 2002, 100:3245–3252. Kallistatin, an inhibitory serpin and a kallikrein inhibitor, exerts an in vitro and in vivo antiangiogenic effect.
Célérier J, Cruz A, Lamandé N, et al.: Angiotensinogen and its cleaved derivatives inhibit angiogenesis. Hypertension 2002, 39:224–228. Discovery of an in vitro and in vivo antiangiogenic effect of AGT. des(Ang I)AGT, for which up to now a role was assigned, shares this function. The antiangiogenic effect of these molecules is most likely due to their serpin structure.
Naftilan AJ, Zuo WM, Ingelfinger JR, et al.: Localization and differential regulation of angiotensinogen mRNA expression in the vessel wall. J Clin Invest 1991, 87:1300–1311.
Saye JA, Lynch KR, Peach MJ: Changes in angiotensinogen messenger RNA in differentiating 3T3-F442A adipocytes. Hypertension 1990, 15:867–871.
Massiera F, Bloch-Faure M, Ceiler D, et al.: Adipose angiotensinogen is involved in adipose tissue growth and blood pressure regulation. FASEB J 2001, 15:2727–2729.
Rupnick MA, Panigrahy D, Zhang CY, et al.: Adipose tissue mass can be regulated through the vasculature. Proc Natl Acad Sci U S A 2002, 98:10730–10735.
Stornetta RL, Hawelu-Johnson CL, Guyenet PG, Lynch KR: Astrocytes synthesize angiotensinogen in brain. Science 1988, 242:1444–1446.
Morimoto S, Cassel MD, Belt TG, et al.: Elevated blood pressure in transgenic mice with brain-specific expression of human angiotensinogen driven by the glial fibrillary acidic protein promoter. Circ Res 2001, 89:365–372.
Kakinuma Y, Hama H, Sugiyama F, et al.: Impaired blood-brain barrier function in angiotensinogen-deficient mice. Nat Med 1998, 4:1078–1080. Discovery of a totally new role of astrocyte AGT. AGT produced by astrocytes is required for functional maintainance of the bloodbrain barrier.
Yanai K, Saito T, Kakinuma Y, et al.: Renin-dependent cardiovascular functions and renin-independent blood-brain barrier functions revealed by renin-deficient mice. J Biol Chem 2000, 275:5–8.
Le Noble FA, Kessels-van Wylick LC, Hacking WJ, et al.: The role of angiotensin II and prostaglandins in arcade formation in a developing microvascular network. J Vasc Res 1996, 33:480–488.
Machado RD, Santos RA, Andrade SP: Opposing actions of angiotensins on angiogenesis. Life Sci 2000, 66:67–76.
Amaral SL, Linderman JR, Morse MM, Greene AS: Angiogenesis induced by electrical stimulation is mediated by angiotensin II and VEGF. Microcirculation 2001, 8:57–67.
Green AS, Amaral SL: Microvascular angiogenesis and the renin-angiotensin system. Cur Hypertens Rep 2002, 4:56–62. A complete and comprehensive review on the connections between the renin-angiotensin system and other growth factor pathways involved in physiologic and pathologic angiogenesis and rarefaction.
Emanueli C, Madeddu P: Renin-angiotensin and kallikreinkinin systems coordinately modulate angiogenesis. Hypertension 2002, 39:224–228.
Emanueli C, Salis MB, Stacca T, et al.: Rescue of impaired angiogenesis in spontaneously hypertensive rats by intramuscular human tissue kallikrein gene transfer. Hypertension 2001, 38:136–141.
Silvestre JS, Bergaya S, Tamarat R, et al.: Proangiogenic effect of angiotensin-converting enzyme inhibition is medited by the bradykinine B(2) receptor pathway. Circ Res 2001, 89:678–683.
Colman RW, Jameson BA, Lin Y, et al.: Domain 5 of high molecular weight kininogen (kininostatin) down-regulates endothelial cell proliferation and migration and inhibits angiogenesis. Blood 2000, 95:543–550.
Fabre JE, Rivard A, Magner M, et al.: Tissue inhibition of angiotensin-converting enzyme activity stimulates angiogenesis in vivo. Circulation 1999, 99:3043–3049. The ACE inhibitor quinapril, but not captopril, promotes angiogenesis in a model of rabbit hind limb ischemia. This study suggests that ACE inhibition may be potentially useful for therapeutic angiogenesis.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Corvol, P., Lamandé, N., Cruz, A. et al. Inhibition of angiogenesis: A new function for angiotensinogen and des(angiotensin I)angiotensinogen. Current Science Inc 5, 149–154 (2003). https://doi.org/10.1007/s11906-003-0072-3
Issue Date:
DOI: https://doi.org/10.1007/s11906-003-0072-3