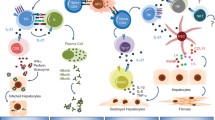
Abstract
Purpose of Review
This review aims to elucidate the multifaceted role of the tumor suppressor protein p53 in the context of HIV infection. We explore how p53, a pivotal regulator of cellular processes, interacts with various facets of the HIV life cycle. Understanding these interactions could provide valuable insights into potential therapeutic interventions and the broader implications of p53 in viral infections.
Recent Findings
Recent research has unveiled a complex interplay between p53 and HIV. Several reports have highlighted the involvement of p53 in restricting the replication of HIV within both immune and nonimmune cells. Various mechanisms have been suggested to unveil how p53 enforces this restriction on HIV replication. However, HIV has developed strategies to manipulate p53, benefiting its replication and evading host defenses.
Summary
In summary, p53 plays a multifaceted role in HIV infection, impacting viral replication and disease progression. Recent findings underscore the importance of understanding the intricate interactions between p53 and HIV for the development of innovative therapeutic approaches. Manipulating p53 pathways may offer potential avenues to suppress viral replication and ameliorate immune dysfunction, ultimately contributing to the management of HIV/AIDS. Further research is warranted to fully exploit the therapeutic potential of p53 in the context of HIV infection.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Hafner A, Bulyk ML, Jambhekar A, Lahav G. The multiple mechanisms that regulate p53 activity and cell fate. Nat Rev Mol Cell Biol. 2019;20(4):199–210.
Lane DP. Cancer p53, guardian of the genome. Nature. 1992;358(6381):15–6.
Lane DP, Crawford LV. T antigen is bound to a host protein in SV40-transformed cells. Nature. 1979;278(5701):261–3.
Linzer DI, Levine AJ. Characterization of a 54K Dalton cellular SV40 tumor antigen present in SV40-transformed cells and uninfected embryonal carcinoma cells. Cell. 1979;17(1):43–52.
Smith AE, Smith R, Paucha E. Characterization of different tumor antigens present in cells transformed by simian virus 40. Cell. 1979;18(2):335–46.
Baker SJ, Fearon ER, Nigro JM, Hamilton SR, Preisinger AC, Jessup JM, et al. Chromosome 17 deletions and p53 gene mutations in colorectal carcinomas. Science. 1989;244(4901):217–21.
Kruse JP, Gu W. Modes of p53 regulation. Cell. 2009;137(4):609–22.
Laptenko O, Prives C. Transcriptional regulation by p53: one protein, many possibilities. Cell Death Differ. 2006;13(6):951–61.
Farnebo M, Bykov VJ, Wiman KG. The p53 tumor suppressor: a master regulator of diverse cellular processes and therapeutic target in cancer. Biochem Biophys Res Commun. 2010;396(1):85–9.
Haupt Y, Maya R, Kazaz A, Oren M. Mdm2 promotes the rapid degradation of p53. Nature. 1997;387(6630):296–9.
Momand J, Wu HH, Dasgupta G. MDM2–master regulator of the p53 tumor suppressor protein. Gene. 2000;242(1–2):15–29.
Zhang Y, Xiong Y, Yarbrough WG. ARF promotes MDM2 degradation and stabilizes p53: ARF-INK4a locus deletion impairs both the Rb and p53 tumor suppression pathways. Cell. 1998;92(6):725–34.
Kurki S, Peltonen K, Latonen L, Kiviharju TM, Ojala PM, Meek D, et al. Nucleolar protein NPM interacts with HDM2 and protects tumor suppressor protein p53 from HDM2-mediated degradation. Cancer Cell. 2004;5(5):465–75.
Leng RP, Lin Y, Ma W, Wu H, Lemmers B, Chung S, et al. Pirh2, a p53-induced ubiquitin-protein ligase, promotes p53 degradation. Cell. 2003;112(6):779–91.
Chen D, Kon N, Li M, Zhang W, Qin J, Gu W. ARF-BP1/Mule is a critical mediator of the ARF tumor suppressor. Cell. 2005;121(7):1071–83.
Dornan D, Wertz I, Shimizu H, Arnott D, Frantz GD, Dowd P, et al. The ubiquitin ligase COP1 is a critical negative regulator of p53. Nature. 2004;429(6987):86–92.
Huang Q, Raya A, DeJesus P, Chao SH, Quon KC, Caldwell JS, et al. Identification of p53 regulators by genome-wide functional analysis. Proc Natl Acad Sci U S A. 2004;101(10):3456–61.
Vogelstein B, Lane D, Levine AJ. Surfing the p53 network. Nature. 2000;408(6810):307–10.
Balint EE, Vousden KH. Activation and activities of the p53 tumour suppressor protein. Br J Cancer. 2001;85(12):1813–23.
Gu W, Roeder RG. Activation of p53 sequence-specific DNA binding by acetylation of the p53 C-terminal domain. Cell. 1997;90(4):595–606.
Pearson M, Carbone R, Sebastiani C, Cioce M, Fagioli M, Saito S, et al. PML regulates p53 acetylation and premature senescence induced by oncogenic Ras. Nature. 2000;406(6792):207–10.
Samuels-Lev Y, O’Connor DJ, Bergamaschi D, Trigiante G, Hsieh JK, Zhong S, et al. ASPP proteins specifically stimulate the apoptotic function of p53. Mol Cell. 2001;8(4):781–94.
Nikolaev AY, Li M, Puskas N, Qin J, Gu W. Parc: a cytoplasmic anchor for p53. Cell. 2003;112(1):29–40.
Huang YF, Wee S, Gunaratne J, Lane DP, Bulavin DV. Isg15 controls p53 stability and functions. Cell Cycle. 2014;13(14):2200–10.
Hainaut P, Hollstein M. p53 and human cancer: the first ten thousand mutations. Adv Cancer Res. 2000;77:81–137.
Munoz-Fontela C, Pazos M, Delgado I, Murk W, Mungamuri SK, Lee SW, et al. p53 serves as a host antiviral factor that enhances innate and adaptive immune responses to influenza A virus. J Immunol. 2011;187(12):6428–36.
Munoz-Fontela C, Macip S, Martinez-Sobrido L, Brown L, Ashour J, Garcia-Sastre A, et al. Transcriptional role of p53 in interferon-mediated antiviral immunity. J Exp Med. 2008;205(8):1929–38.
Pampin M, Simonin Y, Blondel B, Percherancier Y, Chelbi-Alix MK. Cross talk between PML and p53 during poliovirus infection: implications for antiviral defense. J Virol. 2006;80(17):8582–92.
Lazo PA, Santos CR. Interference with p53 functions in human viral infections, a target for novel antiviral strategies? Rev Med Virol. 2011;21(5):285–300.
Li X, Zhang W, Liu Y, Xie J, Hu C, Wang X. Role of p53 in pseudorabies virus replication, pathogenicity, and host immune responses. Vet Res. 2019;50(1):9.
Charni-Natan M, Solomon H, Rotter V. p53 and the viral connection: back into the future (double dagger). Cancers (Basel). 2018;10(6):178.
Sato Y, Tsurumi T. Genome guardian p53 and viral infections. Rev Med Virol. 2013;23(4):213–20.
Maruzuru Y, Fujii H, Oyama M, Kozuka-Hata H, Kato A, Kawaguchi Y. Roles of p53 in herpes simplex virus 1 replication. J Virol. 2013;87(16):9323–32.
Maruzuru Y, Koyanagi N, Takemura N, Uematsu S, Matsubara D, Suzuki Y, et al. p53 is a host cell regulator during herpes simplex encephalitis. J Virol. 2016;90(15):6738–45.
Casavant NC, Luo MH, Rosenke K, Winegardner T, Zurawska A, Fortunato EA. Potential role for p53 in the permissive life cycle of human cytomegalovirus. J Virol. 2006;80(17):8390–401.
Xu D, Du Q, Han C, Wang Z, Zhang X, Wang T, et al. p53 signaling modulation of cell cycle arrest and viral replication in porcine circovirus type 2 infection cells. Vet Res. 2016;47(1):120.
Yaseen MM, Yaseen MM, Alqudah MA. Broadly neutralizing antibodies: an approach to control HIV-1 infection. Int Rev Immunol. 2017;36(1):31–40.
Alqudah MAY, Yaseen MMM, Yaseen MMS. HIV-1 strategies to overcome the immune system by evading and invading innate immune system. HIV AIDS Rev. 2016;15(1):1–12.
Yaseen MM, Abuharfeil NM, Darmani H. Myeloid-derived suppressor cells and the pathogenesis of human immunodeficiency virus infection. Open Biol. 2021;11(11):210216.
Mohammad Yaseen M, Mohammad Abuharfeil N, Darmani H. T-cell evasion and invasion during HIV-1 infection: the role of HIV-1 Tat protein. Cell Immunol. 2022;377:104554.
Yaseen MM, Abuharfeil NM, Homa D. Anatomical distribution of myeloid-derived suppressor cells during HIV infection. Viral Immunol. 2021;10:673–8.
Abuharfeil NM, Yaseen MM, Alsheyab FM. Harnessing antibody-dependent cellular cytotoxicity to control HIV-1 infection. ACS Infect Dis. 2019;5(2):158–76.
Yaseen MM, Abuharfeil NM, Yaseen MM, Shabsoug BM. The role of polymorphonuclear neutrophils during HIV-1 infection. Arch Virol. 2018;163(1):1–21.
Genini D, Sheeter D, Rought S, Zaunders JJ, Susin SA, Kroemer G, et al. HIV induces lymphocyte apoptosis by a p53-initiated, mitochondrial-mediated mechanism. FASEB J. 2001;15(1):5–6.
Imbeault M, Ouellet M, Tremblay MJ. Microarray study reveals that HIV-1 induces rapid type-I interferon-dependent p53 mRNA up-regulation in human primary CD4+ T cells. Retrovirology. 2009;6:5.
Yoon CH, Kim SY, Byeon SE, Jeong Y, Lee J, Kim KP, et al. p53-derived host restriction of HIV-1 replication by protein kinase R-mediated Tat phosphorylation and inactivation. J Virol. 2015;89(8):4262–80.
• Mukerjee R, Claudio PP, Chang JR, Del Valle L, Sawaya BE. Transcriptional regulation of HIV-1 gene expression by p53. Cell Cycle. 2010;9(22):4569–78. This study investigates the molecular mechanisms behind p53’s negative involvement in the transcriptional regulation of HIV-1. The research reveals that p53, when overexpressed, hinders the phosphorylation of a key RNA polymerase II site, resulting in a stall in transcriptional elongation within the HIV-1 LTR.
Duan L, Ozaki I, Oakes JW, Taylor JP, Khalili K, Pomerantz RJ. The tumor suppressor protein p53 strongly alters human immunodeficiency virus type 1 replication. J Virol. 1994;68(7):4302–13.
Bargonetti J, Chicas A, White D, Prives C. p53 represses Sp1 DNA binding and HIV-LTR directed transcription. Cell Mol Biol (Noisy-le-grand). 1997;43(7):935–49.
Li CJ, Wang C, Friedman DJ, Pardee AB. Reciprocal modulations between p53 and Tat of human immunodeficiency virus type 1. Proc Natl Acad Sci U S A. 1995;92(12):5461–4.
Perfettini JL, Castedo M, Roumier T, Andreau K, Nardacci R, Piacentini M, et al. Mechanisms of apoptosis induction by the HIV-1 envelope. Cell Death Differ. 2005;12(Suppl 1):916–23.
Cummins NW, Badley AD. Mechanisms of HIV-associated lymphocyte apoptosis: 2010. Cell Death Dis. 2010;1:e99.
Bakhanashvili M. p53 enhances the fidelity of DNA synthesis by human immunodeficiency virus type 1 reverse transcriptase. Oncogene. 2001;20(52):7635–44.
Bakhanashvili M, Novitsky E, Lilling G, Rahav G. P53 in cytoplasm may enhance the accuracy of DNA synthesis by human immunodeficiency virus type 1 reverse transcriptase. Oncogene. 2004;23(41):6890–9.
Hu WS, Hughes SH. HIV-1 reverse transcription. Cold Spring Harb Perspect Med. 2012;2(10):a006882.
Yaseen MM, Abuharfeil NM, Alqudah MA, Yaseen MM. Mechanisms and factors that drive extensive human immunodeficiency virus type-1 hypervariability: an overview. Viral Immunol. 2017;30(10):708–26.
• Shi B, Sharifi HJ, DiGrigoli S, Kinnetz M, Mellon K, Hu W, et al. Inhibition of HIV early replication by the p53 and its downstream gene p21. Virol J. 2018;15(1):53. This reference explores the inhibitory role of the tumor suppressor protein p53 and its downstream effector gene p21 in the early stages of HIV replication. It likely investigates how these cellular factors interfere with key processes such as viral transcription or integration, shedding light on potential mechanisms for suppressing HIV at an early stage of infection.
Gualberto A, Baldwin AS Jr. p53 and Sp1 interact and cooperate in the tumor necrosis factor-induced transcriptional activation of the HIV-1 long terminal repeat. J Biol Chem. 1995;270(34):19680–3.
Zack JA, Arrigo SJ, Weitsman SR, Go AS, Haislip A, Chen IS. HIV-1 entry into quiescent primary lymphocytes: molecular analysis reveals a labile, latent viral structure. Cell. 1990;61(2):213–22.
Korin YD, Zack JA. Nonproductive human immunodeficiency virus type 1 infection in nucleoside-treated G0 lymphocytes. J Virol. 1999;73(8):6526–32.
Kootstra NA, Schuitemaker H. Proliferation-dependent replication in primary macrophages of macrophage-tropic HIV type 1 variants. AIDS Res Hum Retroviruses. 1998;14(4):339–45.
Herbein G, Varin A. The macrophage in HIV-1 infection: from activation to deactivation? Retrovirology. 2010;7:33.
Badia R, Pujantell M, Riveira-Munoz E, Puig T, Torres-Torronteras J, Marti R, et al. The G1/S specific cyclin D2 is a regulator of HIV-1 restriction in non-proliferating cells. PLoS Pathog. 2016;12(8):e1005829.
Valle-Casuso JC, Allouch A, David A, Lenzi GM, Studdard L, Barre-Sinoussi F, et al. p21 restricts HIV-1 in monocyte-derived dendritic cells through the reduction of deoxynucleoside triphosphate biosynthesis and regulation of SAMHD1 antiviral activity. J Virol. 2017;91(23):e01324-17.
Zhang J, Scadden DT, Crumpacker CS. Primitive hematopoietic cells resist HIV-1 infection via p21. J Clin Invest. 2007;117(2):473–81.
Mlcochova P, Sutherland KA, Watters SA, Bertoli C, de Bruin RA, Rehwinkel J, et al. A G1-like state allows HIV-1 to bypass SAMHD1 restriction in macrophages. EMBO J. 2017;36(5):604–16.
Pan X, Baldauf HM, Keppler OT, Fackler OT. Restrictions to HIV-1 replication in resting CD4+ T lymphocytes. Cell Res. 2013;23(7):876–85.
Shan L, Deng K, Gao H, Xing S, Capoferri AA, Durand CM, et al. Transcriptional reprogramming during effector-to-memory transition renders CD4(+) T cells permissive for latent HIV-1 infection. Immunity. 2017;47(4):766-75 e3.
• Akua T, Rahav G, Saragani Y, Hizi A, Bakhanashvili M. Removal of ribonucleotides by p53 protein incorporated during DNA synthesis by HIV-1 reverse transcriptase. AIDS. 2017;31(3):343–53. This reference suggests an intriguing interaction between the host cell’s p53 protein and HIV-1 reverse transcriptase during the process of reverse transcription. Specifically, it delves into the p53 protein’s role in recognizing and removing ribonucleotides that are erroneously incorporated into the viral DNA during HIV-1 replication. Understanding this novel aspect of host-virus interactions may hold significant implications for the development of antiretroviral strategies.
Saragani Y, Hizi A, Rahav G, Zaouch S, Bakhanashvili M. Cytoplasmic p53 contributes to the removal of uracils misincorporated by HIV-1 reverse transcriptase. Biochem Biophys Res Commun. 2018;497(2):804–10.
Vazquez N, Greenwell-Wild T, Marinos NJ, Swaim WD, Nares S, Ott DE, et al. Human immunodeficiency virus type 1-induced macrophage gene expression includes the p21 gene, a target for viral regulation. J Virol. 2005;79(7):4479–91.
Elahi S, Weiss RH, Merani S. Atorvastatin restricts HIV replication in CD4+ T cells by upregulation of p21. AIDS. 2016;30(2):171–83.
Kinnetz M, Alghamdi F, Racz M, Hu W, Shi B. The impact of p53 on the early stage replication of retrovirus. Virol J. 2017;14(1):151.
Leng J, Ho HP, Buzon MJ, Pereyra F, Walker BD, Yu XG, et al. A cell-intrinsic inhibitor of HIV-1 reverse transcription in CD4(+) T cells from elite controllers. Cell Host Microbe. 2014;15(6):717–28.
Allouch A, David A, Amie SM, Lahouassa H, Chartier L, Margottin-Goguet F, et al. p21-mediated RNR2 repression restricts HIV-1 replication in macrophages by inhibiting dNTP biosynthesis pathway. Proc Natl Acad Sci U S A. 2013;110(42):E3997-4006.
Allouch A, David A, Amie SM, Lahouassa H, Chartier L, Margottin-Goguet F, et al. Reply to Pauls et al. p21 is a master regulator of HIV replication in macrophages through dNTP synthesis block. Proc Natl Acad Sci U S A. 2014;111(14):1325–6.
Schott K, Fuchs NV, Derua R, Mahboubi B, Schnellbacher E, Seifried J, et al. Dephosphorylation of the HIV-1 restriction factor SAMHD1 is mediated by PP2A-B55alpha holoenzymes during mitotic exit. Nat Commun. 2018;9(1):2227.
Cribier A, Descours B, Valadao AL, Laguette N, Benkirane M. Phosphorylation of SAMHD1 by cyclin A2/CDK1 regulates its restriction activity toward HIV-1. Cell Rep. 2013;3(4):1036–43.
Breton Y, Desrosiers V, Ouellet M, Deshiere A, Torresilla C, Cohen EA, et al. Expression of MDM2 in macrophages promotes the early postentry steps of HIV-1 infection through inhibition of p53. J Virol. 2019;93(7):e01871-18.
Sawaya BE, Khalili K, Mercer WE, Denisova L, Amini S. Cooperative actions of HIV-1 Vpr and p53 modulate viral gene transcription. J Biol Chem. 1998;273(32):20052–7.
Chowdhury IH, Wang XF, Landau NR, Robb ML, Polonis VR, Birx DL, et al. HIV-1 Vpr activates cell cycle inhibitor p21/Waf1/Cip1: a potential mechanism of G2/M cell cycle arrest. Virology. 2003;305(2):371–7.
Chen H, Li C, Huang J, Cung T, Seiss K, Beamon J, et al. CD4+ T cells from elite controllers resist HIV-1 infection by selective upregulation of p21. J Clin Invest. 2011;121(4):1549–60.
Saez-Cirion A, Hamimi C, Bergamaschi A, David A, Versmisse P, Melard A, et al. Restriction of HIV-1 replication in macrophages and CD4+ T cells from HIV controllers. Blood. 2011;118(4):955–64.
de Pablo A, Bogoi R, Bejarano I, Toro C, Valencia E, Moreno V, et al. Short communication: p21/CDKN1A expression shows broad interindividual diversity in a subset of HIV-1 elite controllers. AIDS Res Hum Retroviruses. 2016;32(3):232–6.
Barboric M, Peterlin BM. A new paradigm in eukaryotic biology: HIV Tat and the control of transcriptional elongation. PLoS Biol. 2005;3(2):e76.
Isel C, Karn J. Direct evidence that HIV-1 Tat stimulates RNA polymerase II carboxyl-terminal domain hyperphosphorylation during transcriptional elongation. J Mol Biol. 1999;290(5):929–41.
Wei P, Garber ME, Fang SM, Fischer WH, Jones KA. A novel CDK9-associated C-type cyclin interacts directly with HIV-1 Tat and mediates its high-affinity, loop-specific binding to TAR RNA. Cell. 1998;92(4):451–62.
Zhu Y, Pe’ery T, Peng J, Ramanathan Y, Marshall N, Marshall T, et al. Transcription elongation factor P-TEFb is required for HIV-1 tat transactivation in vitro. Genes Dev. 1997;11(20):2622–32.
White CH, Moesker B, Beliakova-Bethell N, Martins LJ, Spina CA, Margolis DM, et al. Transcriptomic analysis implicates the p53 signaling pathway in the establishment of HIV-1 latency in central memory CD4 T cells in an in vitro model. PLoS Pathog. 2016;12(11):e1006026.
Trypsteen W, Mohammadi P, Van Hecke C, Mestdagh P, Lefever S, Saeys Y, et al. Differential expression of lncRNAs during the HIV replication cycle: an underestimated layer in the HIV-host interplay. Sci Rep. 2016;6:36111.
Trypsteen W, White CH, Mukim A, Spina CA, De Spiegelaere W, Lefever S, et al. Long non-coding RNAs and latent HIV - a search for novel targets for latency reversal. PLoS ONE. 2019;14(11):e0224879.
Sun B, Yang R, Mallardo M. Roles of microRNAs in HIV-1 replication and latency. Microrna. 2016;5(2):120–3.
Ruelas DS, Chan JK, Oh E, Heidersbach AJ, Hebbeler AM, Chavez L, et al. MicroRNA-155 reinforces HIV latency. J Biol Chem. 2015;290(22):13736–48.
Chiang K, Sung TL, Rice AP. Regulation of cyclin T1 and HIV-1 replication by microRNAs in resting CD4+ T lymphocytes. J Virol. 2012;86(6):3244–52.
Huang J, Wang F, Argyris E, Chen K, Liang Z, Tian H, et al. Cellular microRNAs contribute to HIV-1 latency in resting primary CD4+ T lymphocytes. Nat Med. 2007;13(10):1241–7.
Nathans R, Chu CY, Serquina AK, Lu CC, Cao H, Rana TM. Cellular microRNA and P bodies modulate host-HIV-1 interactions. Mol Cell. 2009;34(6):696–709.
Heinson AI, Woo J, Mukim A, White CH, Moesker B, Bosque A, et al. Micro RNA targets in HIV latency: insights into novel layers of latency control. AIDS Res Hum Retroviruses. 2021;37(2):109–21.
Brooks CL, Gu W. p53 ubiquitination: Mdm2 and beyond. Mol Cell. 2006;21(3):307–15.
Kastenhuber ER, Lowe SW. Putting p53 in Context. Cell. 2017;170(6):1062–78.
Kumari R, Kohli S, Das S. p53 regulation upon genotoxic stress: intricacies and complexities. Mol Cell Oncol. 2014;1(3):e969653.
Jung YS, Qian Y, Chen X. Examination of the expanding pathways for the regulation of p21 expression and activity. Cell Signal. 2010;22(7):1003–12.
Osei Kuffour E, Konig R, Haussinger D, Schulz WA, Munk C. ISG15 deficiency enhances HIV-1 infection by accumulating misfolded p53. mBio. 2019;10(4):e01342-19.
Osei Kuffour E, Schott K, Jaguva Vasudevan AA, Holler J, Schulz WA, Lang PA, et al. USP18 (UBP43) abrogates p21-mediated inhibition of HIV-1. J Virol. 2018;92(20):e00592-18.
Menendez D, Anderson CW. p53 vs. ISG15: stop, you’re killing me. Cell Cycle. 2014;13(14):2160–1.
Chicas A, Molina P, Bargonetti J. Mutant p53 forms a complex with Sp1 on HIV-LTR DNA. Biochem Biophys Res Commun. 2000;279(2):383–90.
Wilson KM, He JJ. HIV Nef expression down-modulated GFAP expression and altered glutamate uptake and release and proliferation in astrocytes. Aging Dis. 2023;14(1):152–69.
Harrod R, Nacsa J, Van Lint C, Hansen J, Karpova T, McNally J, et al. Human immunodeficiency virus type-1 Tat/co-activator acetyltransferase interactions inhibit p53Lys-320 acetylation and p53-responsive transcription. J Biol Chem. 2003;278(14):12310–8.
Barillari G, Palladino C, Bacigalupo I, Leone P, Falchi M, Ensoli B. Entrance of the Tat protein of HIV-1 into human uterine cervical carcinoma cells causes upregulation of HPV-E6 expression and a decrease in p53 protein levels. Oncol Lett. 2016;12(4):2389–94.
Longo F, Marchetti MA, Castagnoli L, Battaglia PA, Gigliani F. A novel approach to protein-protein interaction: complex formation between the p53 tumor suppressor and the HIV Tat proteins. Biochem Biophys Res Commun. 1995;206(1):326–34.
Greenway AL, McPhee DA, Allen K, Johnstone R, Holloway G, Mills J, et al. Human immunodeficiency virus type 1 Nef binds to tumor suppressor p53 and protects cells against p53-mediated apoptosis. J Virol. 2002;76(6):2692–702.
Ali A, Farooqui SR, Rai J, Singh J, Kumar V, Mishra R, et al. HIV-1 Nef promotes ubiquitination and proteasomal degradation of p53 tumor suppressor protein by using E6AP. Biochem Biophys Res Commun. 2020;529(4):1038–44.
Acknowledgements
We confirm that we do not need permission for Fig. 1.
Funding
Funding was not received for the study.
Author information
Authors and Affiliations
Contributions
M.M.Y. contributed to writing all sections. N.M.A. contributed to writing the introduction and conclusion. H.D. contributed to writing the introduction. M.M.Y., N.M.A., and H.D. critically reviewed and validated the final version of this paper.
Corresponding author
Ethics declarations
Ethical Approval
Not required.
Competing Interests
The authors declare no competing interests.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yaseen, M.M., Abuharfeil, N.M. & Darmani, H. The Role of p53 in HIV Infection. Curr HIV/AIDS Rep 20, 419–427 (2023). https://doi.org/10.1007/s11904-023-00684-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11904-023-00684-8