Abstract
Quantification of plasma HIV-1 RNA below the limit of FDA-approved assays by a single copy quantitative PCR assays (SCA) has provided significant insights into HIV-1 persistence despite potent antiretroviral therapy as well as a means to assess the impact of therapeutic strategies, such as treatment intensification, on residual viremia. In this review, we discuss insights gained from plasma HIV-1 RNA SCA and highlight the need for additional assays to characterize better the cellular and tissue reservoirs of HIV-1. Accurate, reproducible, and sensitive assays to quantify HIV-1 reservoirs, before and after therapeutic interventions, are essential tools in the quest for a cure of HIV-1 infection.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Dornadula G, Zhang H, VanUitert B, Stern J, Livornese Jr L, Ingerman MJ, et al. Residual HIV-1 RNA in blood plasma of patients taking suppressive highly active antiretroviral therapy. JAMA. 1999;282(17):1627–32.
•• Maldarelli F, Palmer S, King MS, Wiegand A, Polis MA, Mican J, et al. ART suppresses plasma HIV-1 RNA to a stable set point predicted by pretherapy viremia. PLoS Pathog. 2007;3(4):e46. This paper demonstrated that patients on suppressive ART had residual viremia (median, 3.1 copies/mL) that fell to a stable set point and did not decrease significantly between weeks 60 and 110 of suppressive ART.
Havlir DV, Bassett R, Levitan D, Gilbert P, Tebas P, Collier AC, et al. Prevalence and predictive value of intermittent viremia with combination hiv therapy. JAMA. 2001;286(2):171–9.
Schockmel GA, Yerly S, Perrin L. Detection of low HIV-1 RNA levels in plasma. J Acquir Immune Defic Syndr Hum Retrovirol. 1997;14(2):179–83.
Yerly S, Kaiser L, Perneger TV, Cone RW, Opravil M, Chave JP, et al. Time of initiation of antiretroviral therapy: impact on HIV-1 viraemia. The Swiss HIV Cohort Study. AIDS. 2000;14(3):243–9.
Yerly S, Perneger TV, Vora S, Hirschel B, Perrin L. Decay of cell-associated HIV-1 DNA correlates with residual replication in patients treated during acute HIV-1 infection. AIDS. 2000;14(18):2805–12.
•• Palmer S, Wiegand AP, Maldarelli F, Bazmi H, Mican JM, Polis M, et al. New real-time reverse transcriptase-initiated PCR assay with single-copy sensitivity for human immunodeficiency virus type 1 RNA in plasma. J Clin Microbiol. 2003;41(10):4531–6. This paper described the single copy assay, the first technique capable of detecting a single copy of plasma HIV-1 RNA.
Ho DD, Neumann AU, Perelson AS, Chen W, Leonard JM, Markowitz M. Rapid turnover of plasma virions and CD4 lymphocytes in HIV-1 infection. Nature. 1995;373(6510):123–6.
Perelson AS, Neumann AU, Markowitz M, Leonard JM, Ho DD. HIV-1 dynamics in vivo: virion clearance rate, infected cell life-span, and viral generation time. Science. 1996;271(5255):1582–6.
•• Wei X, Ghosh SK, Taylor ME, Johnson VA, Emini EA, Deutsch P, et al. Viral dynamics in human immunodeficiency virus type 1 infection. Nature. 1995;373(6510):117–22. References 8–10 were the first reports describing the decay of plasma HIV-1 RNA after initiating ART. They provided the framework for future decay analyses, and characterize the first and most profound phase of decay.
Gartner S, Markovits P, Markovitz DM, Kaplan MH, Gallo RC, Popovic M. The role of mononuclear phagocytes in HTLV-III/LAV infection. Science. 1986;233(4760):215–9.
•• Perelson AS, Essunger P, Cao Y, Vesanen M, Hurley A, Saksela K, et al. Decay characteristics of HIV-1-infected compartments during combination therapy. Nature. 1997;387(6629):188–91. This study described the second phase of plasma HIV-1 RNA decay during ART, providing evidence of long-lived infected cells in vivo that persist despite suppression of viral replication.
Chun TW, Carruth L, Finzi D, Shen X, DiGiuseppe JA, Taylor H, et al. Quantification of latent tissue reservoirs and total body viral load in HIV-1 infection. Nature. 1997;387(6629):183–8.
Finzi D, Hermankova M, Pierson T, Carruth LM, Buck C, Chaisson RE, et al. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science. 1997;278(5341):1295–300.
•• Wong JK, Hezareh M, Gunthard HF, Havlir DV, Ignacio CC, Spina CA, et al. Recovery of replication-competent HIV despite prolonged suppression of plasma viremia. Science. 1997;278(5341):1291–5. References 13–15 were seminal publications that provide evidence that resting CD4+ T cells harbored latent, inducible provirus, preventing eradication of HIV-1 by ART alone.
•• Palmer S, Maldarelli F, Wiegand A, Bernstein B, Hanna GJ, Brun SC, et al. Low-level viremia persists for at least 7 years in patients on suppressive antiretroviral therapy. Proc Natl Acad Sci U S A. 2008;105(10):3879–84. This study revealed a third phase of HIV-1 RNA decay and a fourth phase of no further decay that persists for at least 7 years following initiation of ART, expanding upon the findings in reference 2.
Pascual-Pareja JF, Martinez-Prats L, Luczkowiak J, Fiorante S, Rubio R, Pulido F, et al. Detection of HIV-1 at between 20 and 49 copies per milliliter by the Cobas TaqMan HIV-1 v2.0 assay is associated with higher pretherapy viral load and less time on antiretroviral therapy. J Clin Microbiol. 2010;48(5):1911–2.
Chun TW, Justement JS, Murray D, Hallahan CW, Maenza J, Collier AC, et al. Rebound of plasma viremia following cessation of antiretroviral therapy despite profoundly low levels of HIV reservoir: implications for eradication. AIDS. 2010;24(18):2803–8.
Gandhi RT BR, Aga E, Albrecht M, Demeter L, Bastow B, Siliciano R, Siliciano J, Eron J, ATCG A5173. No evidence for decay in the latent reservoir in HIV-infected patients receiving intensive enfuvirtide-containing ART. 17th Conference on Retroviruses and Opportunistic Infections; San Francisco; 2010.
Gandhi RT, Zheng L, Bosch RJ, Chan ES, Margolis DM, Read S, et al. The effect of raltegravir intensification on low-level residual viremia in HIV-infected patients on antiretroviral therapy: a randomized controlled trial. PLoS Med. 2010. doi:10.1371/journal.pmed/1000321.
• Chun TW, Murray D, Justement JS, Hallahan CW, Moir S, Kovacs C, et al. Relationship between residual plasma viremia and the size of HIV proviral DNA reservoirs in infected individuals receiving effective antiretroviral therapy. J Infect Dis. 2011;204(1):135–8. This paper showed a direct correlation between the size of CD4+ T-cell reservoir (ie, HIV-1 DNA) and residual viremia; however, no correlation was found between reservoir size and immune activation.
Havlir DV, Koelsch KK, Strain MC, Margot N, Lu B, Ignacio CC, et al. Predictors of residual viremia in HIV-infected patients successfully treated with efavirenz and lamivudine plus either tenofovir or stavudine. J Infect Dis. 2005;191(7):1164–8.
Haim-Boukobza S, Morand-Joubert L, Flandre P, Valin N, Fourati S, Sayon S, et al. Higher efficacy of nevirapine than efavirenz to achieve HIV-1 plasma viral load below 1 copy/ml. AIDS. 2011;25(3):341–4.
McKinnon JE, Arribas JR, Pulido F, Delgado R, Mellors JW. The level of persistent HIV viremia does not increase after successful simplification of maintenance therapy to lopinavir/ritonavir alone. AIDS. 2006;20(18):2331–5.
Swindells S, DiRienzo AG, Wilkin T, Fletcher CV, Margolis DM, Thal GD, et al. Regimen simplification to atazanavir-ritonavir alone as maintenance antiretroviral therapy after sustained virologic suppression. JAMA. 2006;296(7):806–14.
Wilkin TJ, McKinnon JE, DiRienzo AG, Mollan K, Fletcher CV, Margolis DM, et al. Regimen simplification to atazanavir-ritonavir alone as maintenance antiretroviral therapy: final 48-week clinical and virologic outcomes. J Infect Dis. 2009;199(6):866–71.
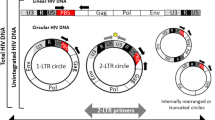
• Buzon MJ, Massanella M, Llibre JM, Esteve A, Dahl V, Puertas MC, et al. HIV-1 replication and immune dynamics are affected by raltegravir intensification of HAART-suppressed subjects. Nat Med. 2010;16(4):460–5. This study showed a transient increase in 2-LTR circles in a fraction of subjects when suppressive ART was intensified with the integrase inhibitor raltegravir, suggesting low-level replication continues despite therapy.
Dinoso JB, Kim SY, Wiegand AM, Palmer SE, Gange SJ, Cranmer L, et al. Treatment intensification does not reduce residual HIV-1 viremia in patients on highly active antiretroviral therapy. Proc Natl Acad Sci U S A. 2009;106(23):9403–8.
Hatano H, Hayes TL, Dahl V, Sinclair E, Lee TH, Hoh R, et al. A randomized, controlled trial of raltegravir intensification in antiretroviral-treated, HIV-infected patients with a suboptimal CD4+ T cell response. J Infect Dis. 2011;203(7):960–8.
McMahon D, Jones J, Wiegand A, Gange SJ, Kearney M, Palmer S, et al. Short-course raltegravir intensification does not reduce persistent low-level viremia in patients with HIV-1 suppression during receipt of combination antiretroviral therapy. Clin Infect Dis. 2010;50(6):912–9.
•• Yukl SA, Shergill AK, McQuaid K, Gianella S, Lampiris H, Hare CB, et al. Effect of raltegravir-containing intensification on HIV burden and T-cell activation in multiple gut sites of HIV-positive adults on suppressive antiretroviral therapy. AIDS. 2010;24(16):2451–60. This study revealed unspliced HIV-1 RNA decrease in gut CD4+ cells during raltegravir intensification, indicating suppressive ART may not completely block replication in tissue compartments.
Gutierrez C DL, Hernandez-Novoa B, Vallejo A, Page C, Lorente R, Madrid N, Palmer S, Munoz-Fernandez MA, Moreno S. Effect of the intensification with a CCR5-antagonist on the decay of the HIV-1 latent reservoir and residual viremia. 17th Conference on Retroviruses & Opportunistic Infections; San Francisco; 2010.
Hilldorfer B LC, McKinnon J, Coombs B, Tenorio A, Fox L, Gandhi R, Ribauldo H, Currier J, Gulick R, Wilkins TJ, Mellors JW. Effects of Maraviroc (MVC) on residual low-level viremia in patients on suppressive Antiretroviral Therapy (ART): Results from ACTG 5256. International AIDS Association, Rome; 2011.
Hunt PW SN, Hayes T, Dahl V, Somsouk M, Funderburg N, Landay AL, Adeyemi O, Shafer R, Clagett B, Rodriguez B, Martin JN, Shacklett B, Palmer S, Lederman MM, Deeks SG The immunomodulatory effects of Maraviroc intensification among ART-suppressed patients with incomplete CD4 recovery. 18th Conference for Retroviruses and Opportunistic Infections; San Francisco; 2011
Wilkin TJ LC, Landay A, Ribaudo H, McKinnon J, Gandhi R, Mellors J, Currier J, Gulick R. Maraviroc (MVC) intensification for suboptimal CD4+ response despite sustained virologic suppression: ACTG 5256. Conference on Retroviruses and Opportunistic Infections; San Francisco; 2010.
Havlir DV, Strain MC, Clerici M, Ignacio C, Trabattoni D, Ferrante P, et al. Productive infection maintains a dynamic steady state of residual viremia in human immunodeficiency virus type 1-infected persons treated with suppressive antiretroviral therapy for five years. J Virol. 2003;77(20):11212–9.
Hammer SM, Ribaudo H, Bassett R, Mellors JW, Demeter LM, Coombs RW, et al. A randomized, placebo-controlled trial of abacavir intensification in HIV-1-infected adults with virologic suppression on a protease inhibitor-containing regimen. HIV Clin Trials. 2010;11(6):312–24.
Pereyra F, Palmer S, Miura T, Block BL, Wiegand A, Rothchild AC, et al. Persistent low-level viremia in HIV-1 elite controllers and relationship to immunologic parameters. J Infect Dis. 2009;200(6):984–90.
Dinoso JB, Kim SY, Siliciano RF, Blankson JN. A comparison of viral loads between HIV-1-infected elite suppressors and individuals who receive suppressive highly active antiretroviral therapy. Clin Infect Dis. 2008;47(1):102–4.
•• Mens H, Kearney M, Wiegand A, Shao W, Schonning K, Gerstoft J, et al. HIV-1 continues to replicate and evolve in patients with natural control of HIV infection. J Virol. 2010;84(24):12971–81. Low-level, residual viremia from elite controls was sequenced in this study and was found to evolve, in contrast to residual viremia observed during suppressive ART that does not show evolution.
Koelsch KK, Liu L, Haubrich R, May S, Havlir D, Gunthard HF, et al. Dynamics of total, linear nonintegrated, and integrated HIV-1 DNA in vivo and in vitro. J Infect Dis. 2008;197(3):411–9.
Liszewski MK, Yu JJ, O'Doherty U. Detecting HIV-1 integration by repetitive-sampling Alu-gag PCR. Methods. 2009;47(4):254–60.
•• Chomont N, El-Far M, Ancuta P, Trautmann L, Procopio FA, Yassine-Diab B, et al. HIV reservoir size and persistence are driven by T cell survival and homeostatic proliferation. Nat Med. 2009;15(8):893–900. This study demonstrated the presence of two distinct HIV-1 DNA reservoirs in the memory T-cell compartment. The reservoir in central memory T cells is maintained by antigen-induced proliferation, whereas the reservoir in transitional memory T cells is maintained by homeostatic proliferation.
Palmer S, Kearney M, Maldarelli F, Halvas EK, Bixby CJ, Bazmi H, et al. Multiple, linked human immunodeficiency virus type 1 drug resistance mutations in treatment-experienced patients are missed by standard genotype analysis. J Clin Microbiol. 2005;43(1):406–13.
Kearney M SJ, Yu S, Shao W, O'Shea A, Rehm C, Poethke C, Mellors J, Coffin J, Maldarelli F. The genetic diversity of HIV-1 in plasma persists despite suppression with ART. Conference on Retroviruses and Opportunistic Infections; San Francisco; 2010.
Rothberg JM, Leamon JH. The development and impact of 454 sequencing. Nat Biotechnol. 2008;26(10):1117–24.
Jabara CJC, Anderson J, Swanstrom R. Accurate sampling and deep sequencing HIV-1 protease using primer ID. Conference on Retroviruses and Opportunistic Infections; Boston; 2011.
•• Bailey JR, Sedaghat AR, Kieffer T, Brennan T, Lee PK, Wind-Rotolo M, et al. Residual human immunodeficiency virus type 1 viremia in some patients on antiretroviral therapy is dominated by a small number of invariant clones rarely found in circulating CD4+ T cells. J Virol. 2006;80(13):6441–57. This report provided extensive genotypic analyses of HIV-1 provirus in circulating CD4+ T cells from patients on suppressive ART. It revealed that the sequences in CD4+ T cells were distinct from those in plasma, providing evidence that residual viremia arises, at least in part, from sources other than circulating CD4+ T cells.
•• Anderson JA, Archin NM, Ince W, Parker D, Wiegand A, Coffin JM, et al. Clonal sequences recovered from plasma from patients with residual HIV-1 viremia and on intensified antiretroviral therapy are identical to replicating viral RNAs recovered from circulating resting CD4+ T cells. J Virol. 2011;85(10):5220–3. This study of two patients on suppressive ART found that HIV-1 sequences isolated from virus outgrowth assays of resting CD4+ T cells matched those of the predominant plasma clone. This is consistent with the hypothesis that residual viremia arises in part from reactivation of latent provirus in resting CD4+ T cells.
Siliciano JD, Siliciano RF. Enhanced culture assay for detection and quantitation of latently infected, resting CD4+ T-cells carrying replication-competent virus in HIV-1-infected individuals. Methods Mol Biol. 2005;304:3–15.
Folks TM, Kessler SW, Orenstein JM, Justement JS, Jaffe ES, Fauci AS. Infection and replication of HIV-1 in purified progenitor cells of normal human bone marrow. Science. 1988;242(4880):919–22.
Kitano K, Abboud CN, Ryan DH, Quan SG, Baldwin GC, Golde DW. Macrophage-active colony-stimulating factors enhance human immunodeficiency virus type 1 infection in bone marrow stem cells. Blood. 1991;77(8):1699–705.
Ruiz ME, Cicala C, Arthos J, Kinter A, Catanzaro AT, Adelsberger J, et al. Peripheral blood-derived CD34+ progenitor cells: CXC chemokine receptor 4 and CC chemokine receptor 5 expression and infection by HIV. J Immunol. 1998;161(8):4169–76.
Steinberg HN, Crumpacker CS, Chatis PA. In vitro suppression of normal human bone marrow progenitor cells by human immunodeficiency virus. J Virol. 1991;65(4):1765–9.
•• Carter CC, Onafuwa-Nuga A, McNamara LA, Riddell JT, Bixby D, Savona MR, et al. HIV-1 infects multipotent progenitor cells causing cell death and establishing latent cellular reservoirs. Nat Med. 2010;16(4):446–51. This report utilized flow cytometry to provide evidence for infection of early progenitor cells in the bone marrow of some patients on suppressive ART, implicating such cells as long-lived reservoirs of HIV-1.
Hagberg L, Cinque P, Gisslen M, Brew BJ, Spudich S, Bestetti A, et al. Cerebrospinal fluid neopterin: an informative biomarker of central nervous system immune activation in HIV-1 infection. AIDS Res Ther. 2010;7:15.
Ambrose Z, Palmer S, Boltz VF, Kearney M, Larsen K, Polacino P, et al. Suppression of viremia and evolution of human immunodeficiency virus type 1 drug resistance in a macaque model for antiretroviral therapy. J Virol. 2007;81(22):12145–55.
DaFonseca S. Purging the HIV-1 reservoir through the disruption of the PD-1 pathway. In: Them TaCHRaStC, editor. International AIDS Society, Rome; 2011.
•• Josefsson L, King MS, Makitalo B, Brannstrom J, Shao W, Maldarelli F, et al. Majority of CD4+ T cells from peripheral blood of HIV-1-infected individuals contain only one HIV DNA molecule. Proc Natl Acad Sci U S A. 2011;108(27):11199–204. This study utilized single cell analyses to demonstrate that most HIV-1-infected cells contain a single provirus. Similar single cell analyses of HIV-1 expression are likely to be important for future studies of HIV-1 reservoirs.
Acknowledgment
Supported by award T32 AI065380 from the National Institute of Allergy and Infectious Disease, NIAID, Division of AIDS (University of Pittsburgh CTU Grant 1U01 AI069494-01 and supplement), a Virology Support Laboratory subcontract (204VC009) of the ACTG Central Group Grant (1U01AI068636-01), and by the NCI (SAIC contract 25XS119).
Disclosure
No potential conflicts of interest relevant to this article were reported.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hilldorfer, B.B., Cillo, A.R., Besson, G.J. et al. New Tools for Quantifying HIV-1 Reservoirs: Plasma RNA Single Copy Assays and Beyond. Curr HIV/AIDS Rep 9, 91–100 (2012). https://doi.org/10.1007/s11904-011-0104-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11904-011-0104-6