Abstract
Purpose of Review
Antibody-drug conjugates are a new class of therapeutic agents in the treatment of B cell malignancies. In this review, we summarize the recent developments of polatuzumab vedotin in the treatment of relapsed or refractory diffuse large B cell lymphoma (DLBCL) and follicular lymphoma (FL).
Recent Findings
Polatuzumab vedotin recently received its first FDA approval in combination with bendamustine and rituximab for the treatment of patients with relapsed or refractory DLBCL. Polatuzumab vedotin has been evaluated and is being studied in combinations with chemoimmunotherapy, immunomodulating agents, bispecific antibodies, and venetoclax. These studies have shown promising results in early phase trials.
Summary
While further studies in a larger patient population are needed in order to determine an optimal combination regimen for polatuzumab vedotin, the ongoing trials represent a growing list of potential therapeutic options for the patients with relapsed or refractory NHL and newly diagnosed NHL alike.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Yu B, Liu D. Antibody-drug conjugates in clinical trials for lymphoid malignancies and multiple myeloma. J Hematol Oncol. 2019;12:94.
D'Arena G, Musto P, Cascavilla N, Dell'Olio M, Di Renzo N, Carotenuto M. Quantitative flow cytometry for the differential diagnosis of leukemic B-cell chronic lymphoproliferative disorders. Am J Hematol. 2000;64:275–81.
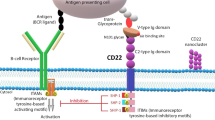
Young RM, Shaffer AL 3rd, Phelan JD, Staudt LM. B-cell receptor signaling in diffuse large B-cell lymphoma. Semin Hematol. 2015;52:77–85.
The Human Protein Atlas. CD79B. 2019, at https://www.proteinatlas.org/ENSG00000007312-CD79B/pathology.)
Polivy™ (Polatuzumab vedotin-piiq) for injection, for intravenous use: Highlights of Prescribing Information. 2019.
Palanca-Wessels MCA, Czuczman M, Salles G, Assouline S, Sehn LH, Flinn I, et al. Safety and activity of the anti-CD79B antibody–drug conjugate polatuzumab vedotin in relapsed or refractory B-cell non-Hodgkin lymphoma and chronic lymphocytic leukaemia: a phase 1 study. Lancet Oncol. 2015;16:704–15.
Hatake K, Kinoshita T, Terui Y, et al. A phase I pharmacokinetic and safety study of polatuzumab vedotin in Japanese patients with relapsed/refractory b-cell non-Hodgkin lymphoma: A comparison with non-Japanese DCS4968g study. J Clin Oncol. 2016;34:e19070–e.
• Morschhauser F, Flinn IW, Advani R, et al. Polatuzumab vedotin or pinatuzumab vedotin plus rituximab in patients with relapsed or refractory non-Hodgkin lymphoma: final results from a phase 2 randomised study (ROMULUS). Lancet Haematol. 2019;6:e254–e65 The ROMULUS trial was the first phase II trial to evaluate the combination of pola with a CD20 monoclonal antibody. With the ORR of 54% and 21% CR rate in the DLBCL population, this trial solidified the pola's role as a viable therapeutic agent in the treatment of R/R DLBCL.
Tobinai K, Klein C, Oya N, Fingerle-Rowson G. A review of Obinutuzumab (GA101), a novel type II anti-CD20 monoclonal antibody, for the treatment of patients with B-cell malignancies. Adv Ther. 2017;34:324–56.
Phillips T, Brunvand M, Chen A, et al. Polatuzumab Vedotin Combined with Obinutuzumab for Patients with Relapsed or Refractory Non-Hodgkin Lymphoma: Preliminary Safety and Clinical Activity of a Phase Ib/II Study. Blood. 2016;128:622.
Sehn LH, Herrera AF, Flowers CR, et al. Polatuzumab Vedotin in Relapsed or Refractory Diffuse Large B-Cell Lymphoma. J Clin Oncol. 2019:JCO.19.00172.
•• Sehn LH, Matasar MJ, Flowers CR, et al. Polatuzumab Vedotin plus Bendamustine with rituximab in relapsed/refractory diffuse large B-cell lymphoma: updated results of a phase Ib/II randomized study. Blood. 2019;134:4081 The randomized phase II trial of this study led to the first FDA approval for pola in combination with bendamustine and rituximab for the treatment of patients with R/R DLBCL who have received at least two prior lines of thearpy.
Sehn LH, Herrera AF, Matasar MJ, et al. Addition of Polatuzumab Vedotin to Bendamustine and Rituximab (BR) Improves Outcomes in Transplant-Ineligible Patients with Relapsed/Refractory (R/R) Diffuse Large B-Cell Lymphoma (DLBCL) Versus BR Alone: Results from a Randomized Phase 2 Study. Blood. 2017;130:2821.
FDA approves polatuzumab vedotin-piiq for diffuse large B-cell lymphoma. 2019. at https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-polatuzumab-vedotin-piiq-diffuse-large-b-cell-lymphoma.
Tilly H, Morschhauser F, Bartlett NL, Mehta A, Salles G, Haioun C, et al. Polatuzumab vedotin in combination with immunochemotherapy in patients with previously untreated diffuse large B-cell lymphoma: an open-label, non-randomised, phase 1b–2 study. Lancet Oncol. 2019;20:998–1010.
• Tilly H, Flowers C, Friedberg JW, et al. POLARIX: a phase 3 study of polatuzumab vedotin (pola) plus R-CHP versus R-CHOP in patients (pts) with untreated DLBCL. J Clin Oncol. 2019;37:TPS7571–TPS The POLARIX trial is the first phase III trial of pola in combiation with chemoimmunotherapy in newly diagnosed DLBCL patientsResults of the POLARIX study are eagerly awaited.
Forero-Torres A, Kolibaba KS, Lamy T, Jones S, Lee C, Sharman J. Polatuzumab Vedotin Combined with Obinutuzumab, Cyclophosphamide, Doxorubicin, and Prednisone (G-CHP) for Patients with Previously Untreated Diffuse Large B-Cell Lymphoma (DLBCL): Preliminary Results of a Phase Ib/II Dose-Escalation Study. Blood. 2016;128:1856.
Morschhauser F, Le Gouill S, Feugier P, et al. Obinutuzumab combined with lenalidomide for relapsed or refractory follicular B-cell lymphoma (GALEN): a multicentre, single-arm, phase 2 study. Lancet Haematol. 2019;6:e429–e37.
Gribben JG, Fowler N, Morschhauser F. Mechanisms of action of Lenalidomide in B-cell non-Hodgkin lymphoma. J Clin Oncol. 2015;33:2803–11.
• Catherine Diefenbach M, Brad S. Kahl M, Lalita Banerjee F, et al. Polatuzumab Vedotin Plus Obinutuzumab and Lenalidomide in Patients With Relapsed/Refractory Follicular Lymphoma: Primary Analysis of the Full Efficacy Population in a Phase Ib/II Trial. American Society of Hematology Annual Meeting 2019;Abstract 126. This is the first trial to study a novel triplet combination with pola, anti-CD20 antibody and an immunomodulating agent in R/R FL, with promising results of tolerability and antitumor activity in the primary analysis of the full efficacy population reported in an abstract format at the 2019 American Society of Hematology annual conference.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
CD: Consulting and research funding: Roche/Genentech. The authors declare no other conflict of interest that is relevant to this review article
Human and Animal Rights and Informed Consent
CD participated in one of the studies referenced in the article.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on T-Cell and Other Lymphoproliferative Malignancies
Rights and permissions
About this article
Cite this article
Choi, Y., Diefenbach, C.S. Polatuzumab Vedotin: a New Target for B Cell Malignancies. Curr Hematol Malig Rep 15, 125–129 (2020). https://doi.org/10.1007/s11899-020-00572-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11899-020-00572-7