Abstract
Purpose of Review
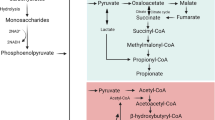
Short-chain fatty acids (SCFAs), the main bacterial fermentation products in the hindgut of hindgut fermenters, are also present in the foregut lumen. We discuss the impact of SCFAs in the duodenal defense mechanisms and in the gastrointestinal (GI) pathogenesis.
Recent Findings
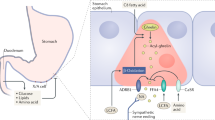
Luminal SCFAs augment the duodenal mucosal defenses via release of serotonin (5-HT) and glucagon-like peptide-2 (GLP-2) from enteroendocrine cells. Released GLP-2 protects the small intestinal mucosa from nonsteroidal anti-inflammatory drug-induced enteropathy. SCFAs are also rapidly absorbed via SCFA transporters and interact with afferent and myenteric nerves. Excessive SCFA signals with 5-HT3 receptor overactivation may be implicated in the pathogenesis of irritable bowel syndrome symptoms. SCFA production exhibits diurnal rhythms with host physiological responses, suggesting that oral SCFA treatment may adjust the GI clocks.
Summary
SCFAs are not only a source of energy but also signaling molecules for the local regulation of the GI tract and systemic regulation via release of gut hormones. Targeting SCFA signals may be a novel therapeutic for GI diseases and metabolic syndrome.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Brown AJ, Goldsworthy SM, Barnes AA, Eilert MM, Tcheang L, Daniels D, et al. The orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J Biol Chem. 2003;278:11312–9.
• Kaji I, Iwanaga T, Watanabe M, Guth PH, Engel E, Kaunitz JD, et al. SCFA transport in rat duodenum. Am J Physiol Gastrointest Liver Physiol. 2015;308:G188–97 This study provides the evidence of rapid, electrogenic SCFA absorption via apical SMCT-1 in the duodenal mucosa.
Hoverstad T, Bjorneklett A, Midtvedt T, Fausa O, Bohmer T. Short-chain fatty acids in the proximal gastrointestinal tract of healthy subjects. Scand J Gastroenterol. 1984;19:1053–8.
Botta GA, Radin L, Costa A, Schito G, Blasi G. Gas-liquid chromatography of the gingival fluid as an aid in periodontal diagnosis. J Periodontal Res. 1985;20:450–7.
Iwanaga T, Kishimoto A. Cellular distributions of monocarboxylate transporters: a review. Biomed Res. 2015;36:279–301.
•• Akiba Y, Inoue T, Kaji I, Higashiyama M, Narimatsu K, Iwamoto K, et al. Short-chain fatty acid sensing in rat duodenum. J Physiol. 2015;593:585–99 This study provides for the first time that luminal SCFAs stimulate duodenal mucosa to enhance mucosal defense via distinct 5-HT and GLP-2 pathways via SCFA receptors.
•• Akiba Y, Maruta K, Narimatsu K, Said H, Kaji I, Kuri A, et al. FFA2 activation combined with ulcerogenic COX inhibition induces duodenal mucosal injury via the 5-HT pathway in rats. Am J Physiol Gastrointest Liver Physiol. 2017;313:G117–28 This study links 5-HT release via FFA2 activation to duodenal mucosal injury with indomethacin treatment, implicating in 5-HT-related IBS symptom generation.
Said H, Akiba Y, Narimatsu K, Maruta K, Kuri A, Iwamoto K, et al. FFA3 activation stimulates duodenal bicarbonate secretion and prevents NSAID-induced enteropathy via the GLP-2 pathway in rats. Dig Dis Sci. 2017;62:1944–52.
Illman RJ, Topping DL, Trimble RP. Effects of food restriction and starvation-refeeding on volatile fatty acid concentrations in the rat. J Nutr. 1986;116:1694–700.
Cummings JH, Pomare EW, Branch WJ, Naylor CP, Macfarlane GT. Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut. 1987;28:1221–7.
Bergman EN. Energy contributions of volatile fatty acids from the gastrointestinal tract in various species. Physiol Rev. 1990;70:567–90.
Skutches CL, Holroyde CP, Myers RN, Paul P, Reichard GA. Plasma acetate turnover and oxidation. J Clin Invest. 1979;64:708–13.
Ostman E, Granfeldt Y, Persson L, Bjorck I. Vinegar supplementation lowers glucose and insulin responses and increases satiety after a bread meal in healthy subjects. Eur J Clin Nutr. 2005;59:983–8.
Brighenti F, Castellani G, Benini L, Casiraghi MC, Leopardi E, Crovetti R, et al. Effect of neutralized and native vinegar on blood glucose and acetate responses to a mixed meal in healthy subjects. Eur J Clin Nutr. 1995;49:242–7.
Ruppin H, Bar-Meir S, Soergel KH, Wood CM, Schmitt MG Jr. Absorption of short-chain fatty acids by the colon. Gastroenterology. 1980;78:1500–7.
Braden B, Adams S, Duan LP, Orth KH, Maul FD, Lembcke B, et al. The [13C]acetate breath test accurately reflects gastric emptying of liquids in both liquid and semisolid test meals. Gastroenterology. 1995;108:1048–55.
Sjölund K, Sandén G, Håkanson R, Sundler F. Endocrine cells in human intestine: an immunocytochemical study. Gastroenterology. 1983;85:1120–30.
Akiba Y, Kaunitz JD. Duodenal luminal chemosensing; acid, ATP, and nutrients. Curr Pharm Des. 2014;20:2760–5.
Akiba Y, Guth PH, Engel E, Nastaskin I, Kaunitz JD. Acid-sensing pathways of rat duodenum. Am J Physiol Gastrointest Liver Physiol. 1999;277:G268–74.
Akiba Y, Ghayouri S, Takeuchi T, Mizumori M, Guth PH, Engel E, et al. Carbonic anhydrases and mucosal vanilloid receptors help mediate the hyperemic response to luminal CO2 in rat duodenum. Gastroenterology. 2006;131:142–52.
Mizumori M, Ham M, Guth PH, Engel E, Kaunitz JD, Akiba Y. Intestinal alkaline phosphatase regulates protective surface microclimate pH in rat duodenum. J Physiol. 2009;587:3651–63.
Engelstoft MS, Egerod KL, Holst B, Schwartz TW. A gut feeling for obesity: 7TM sensors on enteroendocrine cells. Cell Metab. 2008;8:447–9.
Tazoe H, Otomo Y, Karaki S, Kato I, Fukami Y, Terasaki M, et al. Expression of short-chain fatty acid receptor GPR41 in the human colon. BiomedRes. 2009;30:149–56.
Karaki S, Tazoe H, Hayashi H, Kashiwabara H, Tooyama K, Suzuki Y, et al. Expression of the short-chain fatty acid receptor, GPR43, in the human colon. J Mol Histol. 2008;39:135–42.
Akiba Y, Inoue T, Kaji I, Higashiyama M, Guth PH, Engel E, et al. Short-chain fatty acid sensing in rat duodenum. J Physiol. 2014;593:585–99.
•• Wan Saudi WS, Sjöblom M. Short-chain fatty acids augment rat duodenal mucosal barrier function. Exp Physiol. 2017;102:791–803 This study provides that luminal SCFAs rather than IV SCFAs enhance duodenal defense mechanisms.
Wang JH, Inoue T, Higashiyama M, Guth PH, Engel E, Kaunitz JD, et al. Umami receptor activation increases duodenal bicarbonate secretion via glucagon-like peptide-2 release in rats. J Pharmacol Exp Ther. 2011;339:464–73.
Guan X, Karpen HE, Stephens J, Bukowski JT, Niu S, Zhang G, et al. GLP-2 receptor localizes to enteric neurons and endocrine cells expressing vasoactive peptides and mediates increased blood flow. Gastroenterology. 2006;130:150–64.
Kaji I, Iwanaga T, Watanabe M, Guth PH, Engel E, Kaunitz JD, et al. SCFA transport in rat duodenum. Am J Physiol Gastrointest Liver Physiol. 2014;308:G188–97.
Kaji I, Akiba Y, Konno K, Watanabe M, Kimura S, Iwanaga T, et al. Neural FFA3 activation inversely regulates anion secretion evoked by nicotinic ACh receptor activation in rat proximal colon. J Physiol. 2016;594:3339–52.
Rowland KJ, Brubaker PL. The “cryptic” mechanism of action of glucagon-like peptide-2. Am J Physiol Gastrointest Liver Physiol. 2011;301:G1–8.
Jeppesen PB, Gilroy R, Pertkiewicz M, Allard JP, Messing B, O'Keefe SJ. Randomised placebo-controlled trial of teduglutide in reducing parenteral nutrition and/or intravenous fluid requirements in patients with short bowel syndrome. Gut. 2011;60:902–14.
Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet. 2006;368:1696–705.
Inoue T, Wang JH, Higashiyama M, Rudenkyy S, Higuchi K, Guth PH, et al. Dipeptidyl peptidase IV inhibition potentiates amino acid- and bile acid-induced bicarbonate secretion in rat duodenum. Am J Physiol Gastrointest Liver Physiol. 2012;303:G810–6.
Higuchi K, Umegaki E, Watanabe T, Yoda Y, Morita E, Murano M, et al. Present status and strategy of NSAIDs-induced small bowel injury. J Gastroenterol. 2009;44:879–88.
Inoue T, Higashiyama M, Kaji I, Rudenkyy S, Higuchi K, Guth PH, et al. Dipeptidyl peptidase IV inhibition prevents the formation and promotes the healing of indomethacin-induced intestinal ulcers in rats. Dig Dis Sci. 2014;59:1286–95.
Kaji I, Akiba Y, Furuyama T, Adelson DW, Iwamoto K, Watanabe M, et al. Free fatty acid receptor 3 activation suppresses neurogenic motility in rat proximal colon. Neurogastroenterol Motil, in press. 2017.
Fujimiya M, Okumiya K, Kuwahara A. Immunoelectron microscopic study of the luminal release of serotonin from rat enterochromaffin cells induced by high intraluminal pressure. Histochem Cell Biol. 1997;108:105–13.
Kellum JM, Albuquerque FC, Stoner MC, Harris RP. Stroking human jejunal mucosa induces 5-HT release and Cl- secretion via afferent neurons and 5-HT4 receptors. Am J Phys. 1999;277:G515–20.
Braun T, Voland P, Kunz L, Prinz C, Gratzl M. Enterochromaffin cells of the human gut: sensors for spices and odorants. Gastroenterology. 2007;132:1890–901.
Kellum JM, Donowitz M, Cerel A, Wu J. Acid and isoproterenol cause serotonin release by acting on opposite surfaces of duodenal mucosa. J Surg Res. 1984;36:172–6.
Fukumoto S, Tatewaki M, Yamada T, Fujimiya M, Mantyh C, Voss M, et al. Short-chain fatty acids stimulate colonic transit via intraluminal 5-HT release in rats. Am J Physiol Regul Integr Comp Physiol. 2003;284:R1269–76.
Turvill JL, Connor P, Farthing MJ. The inhibition of cholera toxin-induced 5-HT release by the 5-HT3 receptor antagonist, granisetron, in the rat. Br J Pharmacol. 2000;130:1031–6.
Hagbom M, Istrate C, Engblom D, Karlsson T, Rodriguez-Diaz J, Buesa J, et al. Rotavirus stimulates release of serotonin (5-HT) from human enterochromaffin cells and activates brain structures involved in nausea and vomiting. PLoS Pathog. 2011;7:e1002115.
Cubeddu LX. Serotonin mechanisms in chemotherapy-induced emesis in cancer patients. Oncology. 1996;53(Suppl 1):18–25.
Lee KJ, Tack J. Duodenal implications in the pathophysiology of functional dyspepsia. J Neurogastroenterol Motil. 2010;16:251–7.
Beattie DT, Smith JA. Serotonin pharmacology in the gastrointestinal tract: a review. Naunyn Schmiedeberg's Arch Pharmacol. 2008;377:181–203.
Bharucha AE, Camilleri M, Burton DD, Thieke SL, Feuerhak KJ, Basu A, et al. Increased nutrient sensitivity and plasma concentrations of enteral hormones during duodenal nutrient infusion in functional dyspepsia. Am J Gastroenterol. 2014;109:1910–20.
van Boxel OS, ter Linde JJ, Siersema PD, Smout AJ. Role of chemical stimulation of the duodenum in dyspeptic symptom generation. Am J Gastroenterol. 2010;105:803–11.
Glisic R, Koko V, Todorovic V, Drndarevic N, Cvijic G. Serotonin-producing enterochromaffin (EC) cells of gastrointestinal mucosa in dexamethasone-treated rats. Regul Pept. 2006;136:30–9.
Fukudo S, Kinoshita Y, Okumura T, Ida M, Akiho H, Nakashima Y, et al. Ramosetron reduces symptoms of irritable bowel syndrome with diarrhea and improves quality of life in women. Gastroenterology. 2016;150:358–66.
Garsed K, Chernova J, Hastings M, Lam C, Marciani L, Singh G, et al. A randomised trial of ondansetron for the treatment of irritable bowel syndrome with diarrhoea. Gut. 2014;63:1617–25.
Ghoshal UC, Srivastava D, Misra A, Ghoshal U. A proof-of-concept study showing antibiotics to be more effective in irritable bowel syndrome with than without small-intestinal bacterial overgrowth: a randomized, double-blind, placebo-controlled trial. Eur J Gastroenterol Hepatol. 2016;28:281–9.
Pimentel M, Chow EJ, Lin HC. Eradication of small intestinal bacterial overgrowth reduces symptoms of irritable bowel syndrome. Am J Gastroenterol. 2000;95:3503–6.
Bohn L, Storsrud S, Liljebo T, Collin L, Lindfors P, Tornblom H, et al. Diet low in FODMAPs reduces symptoms of irritable bowel syndrome as well as traditional dietary advice: a randomized controlled trial. Gastroenterology. 2015;149:1399–407.
Barreto JC, Smith GS, Tornwall MS, Miller TA. Protective action of oral N-acetylcysteine against gastric injury: role of hypertonic sodium. Am J Physiol. 1993;264:G422–6.
Aihara E, Sasaki Y, Ise F, Kita K, Nomura Y, Takeuchi K. Distinct mechanisms of acid-induced HCO3 - secretion in normal and slightly permeable stomachs. Am J Physiol Gastrointest Liver Physiol. 2006;291:G464–71.
Black JW, Fisher EW, Smith AN. The effects of 5-hydroxytryptamine on gastric secretion in anaesthetized dogs. J Physiol. 1958;141:27–34.
Canfield SP, Spencer JE. The inhibitory effects of 5-hydroxytryptamine on gastric acid secretion by the rat isolated stomach. Br J Pharmacol. 1983;78:123–9.
Kaji I, Akiba Y, Kaunitz JD, Karaki S, Kuwahara A. Differential expression of short-chain fatty acid receptor FFA2 and FFA3 in foregut. Gastroenterology. 2012;142:S494.
Said HM, Akiba Y, Kaji I, Narimatsu K, Kaunitz JD. FFA2 activation suppresses basal and stimulated gastric acid secretion via 5-HT3 receptor activation in rats. Gastroenterology. 2015;148:S-315.
Lai YC, Ho Y, Huang KH, Tsai LH. Effects of serotonin on acid secretion in isolated rat stomach: the role of 5-HT3 receptors. Chin J Physiol. 2009;52:395–405.
• Segers A, Desmet L, Thijs T, Verbeke K, Tack J, Depoortere I. The circadian clock regulates the diurnal levels of microbial short-chain fatty acids and their rhythmic effects on colon contractility in mice. Acta Physiol (Oxf). 2018:e13193 This study reports diurnal fluctuation of fecal SCFAs synchronized with colonic myenteric neural FFA3 expression, suggesting that luminal SCFA production regulates SCFA receptor expression.
•• Tahara Y, Yamazaki M, Sukigara H, Motohashi H, Sasaki H, Miyakawa H, et al. Gut microbiota-derived short chain fatty acids induce circadian clock entrainment in mouse peripheral tissue. Sci Rep. 2018;8:1395 This study provides a new concept that oral SCFA treatment facilitates peripheral clock adjustment, suggesting that the microbiome and host organs are communicating with circadian rhythmicity.
Funding
This work was supported by a Department of Veterans Affairs Merit Review Award and National Institute of Diabetes and Digestive and Kidney Diseases Grant R01-DK-54221.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Stomach and Duodenum
Rights and permissions
About this article
Cite this article
Iwasaki, M., Akiba, Y. & Kaunitz, J.D. Duodenal Chemosensing of Short-Chain Fatty Acids: Implications for GI Diseases. Curr Gastroenterol Rep 21, 35 (2019). https://doi.org/10.1007/s11894-019-0702-9
Published:
DOI: https://doi.org/10.1007/s11894-019-0702-9