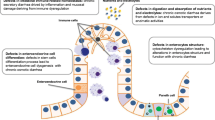
Abstract
Congenital diarrheal disorders (CDD) are a group of rare enteropathies related to specific genetic defects. Infants with these disorders have chronic diarrhea, frequently requiring parenteral nutrition support. Etiologies and prognoses are variable. We propose a new classification of CDD into four groups, taking into account the specific etiology and genetic defect: 1) defects in digestion, absorption, and transport of nutrients and electrolytes; 2) disorders of enterocyte differentiation and polarization; 3) defects of enteroendocrine cell differentiation; and 4) dysregulation of the intestinal immune response. The present review focuses on the recent advances made in understanding the pathophysiology of CDD that could potentially improve the clinical approach to these conditions.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Berni Canani R, Cirillo P, Terrin G. Chronic and intractable diarrhea. In: Guandalini S Ed. Essential Pediatric Gastroenterology Hepatology, and Nutrition. McGraw-Hill Mediacla Publishing Division Chicago 2005; 25–47.
•• Ruemmele FM. Chronic enteropathy: molecular basis. In Gastrointestinal Disorders. Nestlè Nutr Workshop Ser Pediatr Program. 2007; 59:73–88. This article provides an excellent review of molecular mechanisms of chronic enteropathy.
•• Berni Canani R, Terrin G, Cardillo G, et al. Congenital diarrheal disorders: improved understanding of gene defects is leading to advances in intestinal physiology and clinical management. J Pediatr Gastroenterol Nutr. 2010; 50:360–6. This article is an interesting review based on a new classification of congenital diarrhea.
Sherman PM, Mitchell DJ, Cutz E. Neonatal enteropathies: defining the causes of protracted diarrhea of infancy. J Pediatr Gastroenterol Nutr. 2004;38:16–26.
Guarino A, Spagnuolo MI, Russo S, et al. Etiology and risk factors of severe and protracted diarrea. J Pediatr Gastroenterol Nutr. 1995;20:173–8.
Passariello A, Terrin G, Baldassarre ME, et al. Diarrhea in neonatal intensive care unit. World J Gastroenterol. 2010;16:2664–8.
Heyman MB. Lactose intolerance in infants, children, and adolescents. Committee on Nutrition. Pediatrics. 2006;118:1279–86.
Järvelä I, Torniainen S, Kolho KL. Molecular genetics of human lactase deficiencies. Ann Med. 2009;41:568–75.
Torniainen S, Freddara R, Routi T, et al. Four novel mutations in the lactase gene (LCT) underlying congenital lactase deficiency (CLD). BMC Gastroenterol. 2009;9:8.
Robayo-Torres CC, Quezada-Calvillo R, Nichols BL. Disaccharide digestion: clinical and molecular aspects. Clin Gastroenterol Hepatol. 2006;4:276–87.
Nichols BL, Quezada-Calvillo R, Robayo-Torres CC, et al. Mucosal maltase-glucoamylase plays a crucial role in starch digestion and prandial glucose homeostasis of mice. J Nutr. 2009;139:684–90.
•• Venkatasubramanian J, Ao M, Rao MC. Ion transport in the small intestine. Curr Opinion Gatroenterol. 2010; 26:123–8. Interesting review focusing on ions transport mechanisms.
• Xin B, Wang H. Multiple sequence variations in SLC5A1 gene are associated with glucose-galactose malabsorption in a large cohort of Old Order Amish. Clin Genet. 2011;79(1):86–91. doi:10.1111/j.1399-0004.2010.01440.x. The authors of this interesting study report new clinical and molecular insights from a large population of affected subjects.
Gibson PR, Newnham E, Barrett JS, et al. Review article: fructose malabsorption and the bigger picture. Aliment Pharmacol Ther. 2007;25:349–63.
Leturque A, Brot-Laroche E, Le Gall M. GLUT2 mutations, translocation, and receptor function in diet sugar managing. Am J Physiol Endocrinol Metab. 2009;296:E985–92.
•• Dorwart MR, Shcheynikov N, Baker JM, et al. Congenital chloride-losing diarrhea causing mutations in the STAS domain result in misfolding and mistrafficking of SLC26A3. J Biol Chem. 2008;283:8711–22. An important study that sheds light on the role of the STAS domain in the function of the SLC26A3 gene.
Al Makadma AS, Al-Akash SI, Al Dalaan I, et al. Congenital sodium diarrhea in a neonate presenting as acute renal failure. Pediatr Nephrol. 2004;19:905–7.
Leturque A, Brot-Laroche E, Le Gall M. GLUT2 mutations, translocation, and receptor function in diet sugar managing. Am J Physiol Endocrinol Metab. 2009;296:E985–92.
Schmitt S, Küry S, Giraud M, et al. An update on mutations of the SLC39A4 gene in acrodermatitis enteropathica. Hum Mutat. 2009;30:926–33.
Sperandeo MP, Andria G, Sebastio G. Lysinuric protein intolerance: update and extended mutation analysis of the SLC7A7 gene. Hum Mutat. 2008;29:14–21.
Burroughs L, Woolfrey A, Shimamura A. Shwachman-Diamond syndrome: a review of the clinical presentation, molecular pathogenesis, diagnosis, and treatment. Hematol Oncol Clin North Am. 2009;23:233–48.
Cutting GR. Modifier genes in Mendelian disorders: the example of cystic fibrosis. Ann N Y Acad Sci. 2010;1214:57–69. doi:10.1111/j.1749-6632.2010.05879.x.
Holzinger A, Maier EM, Bück C, et al. Mutations in the proenteropeptidase gene are the molecular cause of congenital enteropeptidase deficiency. Am J Hum Genet. 2002;70:20–5.
Zamel R, Khan R, Pollex RL, Hegele RA. Abetalipoproteinemia: two case reports and literature review. Orphanet J Rare Dis. 2008;3:19.
Marcil V, Peretti N, Delvin E, Levy E. Digestive and absorptive processes of lipids. Gastroenterol Clin Biol. 2004;28:1257–66.
Shneider BL. Intestinal bile acid transport: biology, physiology, and pathophysiology. J Pediatr Gastroenterol Nutr. 2001;32:407–17.
• Müller T, Hess MW, Schiefermeier N, et al. MYO5B mutations cause microvillus inclusion disease and disrupt epithelial cell polarity. Nat Genet. 2008; 40:1163–5. This well-designed study suggests a defect in MYO5B as a cause of disease.
Iancu TC, Mahajnah M, Manov I, Shaoul R. Microvillous inclusion disease: ultrastructural variability. Ultrastruct Pathol. 2007;31:173–88.
• Sivagnanam M, Mueller JL, Lee H, et al. Identification of EpCAM as the gene for congenital tufting enteropathy. Gastroenterology. 2008;135:429–37. This is the first article describing the gene responsible for the disease.
Wang J, Cortina G, Wu SV, et al. Mutant neurogenin-3 in congenital malabsorptive diarrhea. N Engl J Med. 2006;355:270–80.
Bjerknes M, Cheng H. Neurogenin 3 and the enteroendocrine cell lineage in the adult mouse small intestinal epithelium. Dev Biol. 2006;300:722–35.
Blanco Quirós A, Arranz Sanz E, Bernardo Ordiz D, Garrote Adrados JA. From autoimmune enteropathy to the IPEX (immune dysfunction, polyendocrinopathy, enteropathy, X-linked) syndrome. Allergol Immunopathol. 2009;37:208–15.
•• Sharma R, Ju ST. Genetic control of the inflammatory T-cell response in regulatory T-cell deficient scurfy mice. Clin Immunol 2010;136:162–9. The authors provide a well-written review on the role of regulatory T cells.
Disclosure
Conflicts of interest: R.B. Canani—none; G. Terrin—none.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Canani, R.B., Terrin, G. Recent Progress in Congenital Diarrheal Disorders. Curr Gastroenterol Rep 13, 257–264 (2011). https://doi.org/10.1007/s11894-011-0188-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11894-011-0188-6