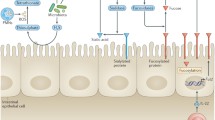
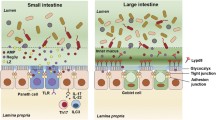
Abstract
Sampling of intestinal pathogens and commensals is an important aspect of the gut immune system, and is accomplished through the action of specialized epithelial M cells. Although their sampling abilities have been appreciated for decades, few molecular details of their development or function are known. This review discusses several recent advances in our understanding of these cells, including signals controlling their development, the mechanisms they use for taking up microbes, and their exploitation by certain pathogens. Future research directions are discussed, including development of oral vaccines.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Simon GL, Gorbach SL: The human intestinal microflora. Dig Dis Sci 1986, 31(9 Suppl):147S–162S.
Fagarasan S, Muramatsu M, Suzuki K, et al.: Critical roles of activation-induced cytidine deaminase in the homeostasis of gut flora. Science 2002, 298:1424–1427.
Peterson DA, McNulty NP, Guruge JL, Gordon JI: IgA response to symbiotic bacteria as a mediator of gut homeostasis. Cell Host Microbe 2007, 2:328–339.
Slack E, Hapfelmeier S, Stecher B, et al.: Innate and adaptive immunity cooperate flexibly to maintain host-microbiota mutualism. Science 2009, 325:617–620.
Niess JH, Brand S, Gu X, et al.: CX3CR1-mediated dendritic cell access to the intestinal lumen and bacterial clearance. Science 2005, 307:254–258.
Bockman DE, Cooper MD: Pinocytosis by epithelium associated with lymphoid follicles in the bursa of Fabricius, appendix, and Peyer’s patches. An electron microscopic study. Am J Anat 1973, 136:455–477.
Kohbata S, Yokoyama H, Yabuuchi E: Cytopathogenic effect of Salmonella typhi GIFU 10007 on M cells of murine ileal Peyer’s patches in ligated ileal loops: an ultrastructural study. Microbiol Immunol 1986, 30:1225–1237.
Autenrieth IB, Firsching R: Penetration of M cells and destruction of Peyer’s patches by Yersinia enterocolitica: an ultrastructural and histological study. J Med Microbiol 1996, 44:285–294.
Wolf JL, Rubin DH, Finberg R, et al.: Intestinal M cells: a pathway for entry of reovirus into the host. Science 1981, 212:471–472.
Jepson MA, Clark MA, Hirst BH: M cell targeting by lectins: a strategy for mucosal vaccination and drug delivery. Adv Drug Deliv Rev 2004, 56:511–525.
Clark MA, Jepson MA, Simmons NL, et al.: Differential expression of lectin-binding sites defines mouse intestinal M-cells. J Histochem Cytochem 1993, 41:1679–1687.
• Nochi T, Yuki Y, Matsumura A, et al.: A novel M cell-specific carbohydrate-targeted mucosal vaccine effectively induces antigen-specific immune responses. J Exp Med 2007, 204:2789–2796. A novel M cell-specific antibody was developed and used to successfully target antigen to M cells and induce an immune response.
Giannasca PJ, Giannasca KT, Leichtner AM, Neutra MR: Human intestinal M cells display the sialyl Lewis A antigen. Infect Immun 1999, 67:946–953.
Pappo J, Ermak TH: Uptake and translocation of fluorescent latex particles by rabbit Peyer’s patch follicle epithelium: a quantitative model for M cell uptake. Clin Exp Immunol 1989, 76:144–148.
Owen RL: Sequential uptake of horseradish peroxidase by lymphoid follicle epithelium of Peyer’s patches in the normal unobstructed mouse intestine: an ultrastructural study. Gastroenterology 1977, 72:440–451.
Frey A, Giannasca KT, Weltzin R, et al.: Role of the glycocalyx in regulating access of microparticles to apical plasma membranes of intestinal epithelial cells: implications for microbial attachment and oral vaccine targeting. J Exp Med 1996, 184:1045–1059.
Isberg RR, Leong JM: Multiple beta 1 chain integrins are receptors for invasin, a protein that promotes bacterial penetration into mammalian cells. Cell 1990, 60:861–871.
Clark MA, Hirst BH, Jepson MA: M-cell surface beta1 integrin expression and invasin-mediated targeting of Yersinia pseudotuberculosis to mouse Peyer’s patch M cells. Infect Immun 1998, 66:1237–1243.
Karapetian O, Shakhov AN, Kraehenbuhl JP, Acha-Orbea H: Retroviral infection of neonatal Peyer’s patch lymphocytes: the mouse mammary tumor virus model. J Exp Med 1994, 180:1511–1516.
Golovkina TV, Shlomchik M, Hannum L, Chervonsky A: Organogenic role of B lymphocytes in mucosal immunity. Science 1999, 286:1965–1968.
Fotopoulos G, Harari A, Michetti P, et al.: Transepithelial transport of HIV-1 by M cells is receptor-mediated. Proc Natl Acad Sci U S A 2002, 99:9410–9414.
Sicinski P, Rowinski J, Warchol JB, et al.: Poliovirus type 1 enters the human host through intestinal M cells. Gastroenterology 1990, 98:56–58.
Helander A, Silvey KJ, Mantis NJ, et al.: The viral sigma1 protein and glycoconjugates containing alpha2-3-linked sialic acid are involved in type 1 reovirus adherence to M cell apical surfaces. J Virol 2003, 77:7964–7977.
Roy MJ, Varvayanis M: Development of dome epithelium in gut-associated lymphoid tissues: association of IgA with M cells. Cell Tissue Res, 1987, 248:645–651.
Mantis NJ, Cheung MC, Chintalacharuvu KR, et al.: Selective adherence of IgA to murine Peyer’s patch M cells: evidence for a novel IgA receptor. J Immunol 2002, 169:1844–1851.
• Terahara K, Yoshida M, Igarashi O, et al.: Comprehensive gene expression profiling of Peyer’s patch M cells, villous M-like cells, and intestinal epithelial cells. J Immunol 2008, 180:7840–7846. This article describes use of a microarray to compare gene expression between intestinal epithelial cells, PP M cells, and villous M cells.
Yu S, Lowe AW: The pancreatic zymogen granule membrane protein, GP2, binds Escherichia coli Type 1 fimbriae. BMC Gastroenterol 2009, 9:58.
•• Hase K, Kawano K, Nochi T, et al.: Uptake through glycoprotein 2 of FimH(+) bacteria by M cells initiates mucosal immune response. Nature 2009, 462:226–230. This study identified GP2 as a receptor used by M cells for sampling of bacteria.
Mach J, Hshieh T, Hsieh D, et al.: Development of intestinal M cells. Immunol Rev 2005, 206:177–189.
Iwatsuki H, Ogawa C, Suda M: Vimentin-positive cells in the villus epithelium of the rabbit small intestine. Histochem Cell Biol 2002, 117:363–370.
Jang MH, Kweon MN, Iwatani K, et al.: Intestinal villous M cells: an antigen entry site in the mucosal epithelium. Proc Natl Acad Sci U S A 2004, 101:6110–6115.
De Togni P, Goellner J, Ruddle NH, et al.: Abnormal development of peripheral lymphoid organs in mice deficient in lymphotoxin. Science 1994, 264:703–707.
Kuprash DV, Tumanov AV, Liepinsh DJ, et al.: Novel tumor necrosis factor-knockout mice that lack Peyer’s patches. Eur J Immunol 2005, 35:1592–1600.
Tumanov AV, Kuprash DV, Mach JA, et al.: Lymphotoxin and TNF produced by B cells are dispensable for maintenance of the follicle-associated epithelium but are required for development of lymphoid follicles in the Peyer’s patches. J Immunol 2004, 173:86–91.
Wang J, Lopez-Fraga M, Rynko A, Lo DD: TNFR and LTbetaR agonists induce follicle-associated epithelium and M cell specific genes in rat and human intestinal epithelial cells. Cytokine 2009, 47:69–76.
•• Knoop KA, Kumar N, Butler BR, et al.: RANKL is necessary and sufficient to initiate development of antigen-sampling M cells in the intestinal epithelium. J Immunol 2009, 183:5738–5747. This study uncovered RANK signaling as an important mediator of both PP and villous M cell development.
Leibbrandt A, Penninger JM: RANK/RANKL: regulators of immune responses and bone physiology. Ann N Y Acad Sci 2008, 1143:123–150.
Kong YY, Yoshida H, Sarosi I, et al.: OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature 1999, 397:315–323.
Taylor RT, Patel SR, Lin E, et al.: Lymphotoxin-independent expression of TNF-related activation-induced cytokine by stromal cells in cryptopatches, isolated lymphoid follicles, and Peyer’s patches. J Immunol 2007, 178:5659–5667.
• Hsieh EH, Fernandez X, Wang J, et al.: CD137 is required for M cell functional maturation but not lineage commitment. Am J Pathol 2010 (Epub ahead of print). This article describes an intermediate stage of PP M cell differentiation that is dependent on CD137 signaling.
Savidge TC, Smith MW, James PS, Aldred P: Salmonella-induced M-cell formation in germ-free mouse Peyer’s patch tissue. Am J Pathol 1991, 139:177–184.
Borghesi C, Regoli M, Bertelli E, Nicoletti C: Modifications of the follicle-associated epithelium by short-term exposure to a non-intestinal bacterium. J Pathol 1996, 180:326–332.
Smith MW, James PS, Tivey DR: M cell numbers increase after transfer of SPF mice to a normal animal house environment. Am J Pathol 1987, 128:385–389.
Lo D, Tynan W, Dickerson J, et al.: Peptidoglycan recognition protein expression in mouse Peyer’s Patch follicle associated epithelium suggests functional specialization. Cell Immunol 2003, 224:8–16.
Verbrugghe P, Waelput W, Dieriks B, et al.: Murine M cells express annexin V specifically. J Pathol 2006, 209:240–249.
Adachi S, Yoshida H, Kataoka H, Nishikawa S: Three distinctive steps in Peyer’s patch formation of murine embryo. Int Immunol 1997, 9:507–514.
Holmgren J, Czerkinsky C: Mucosal immunity and vaccines. Nat Med 2005, 11(4 Suppl):S45–S53.
Sabin AB: Properties and behavior of orally administered attenuated poliovirus vaccine. J Am Med Assoc 1957, 164:1216–1223.
Chionh YT, Wee JL, Every AL, et al.: M-cell targeting of whole killed bacteria induces protective immunity against gastrointestinal pathogens. Infect Immun 2009, 77:2962–2970.
Iweala OI, Smith DW, Matharu KS, et al.: Vaccine-induced antibody isotypes are skewed by impaired CD4 T cell and invariant NKT cell effector responses in MyD88-deficient mice. J Immunol 2009, 183:2252–2260.
Kotton CN, Hohmann EL: Enteric pathogens as vaccine vectors for foreign antigen delivery. Infect Immun 2004, 72:5535–5547.
Kerneis S, Bogdanova A, Kraehenbuhl JP, Pringault E: Conversion by Peyer’s patch lymphocytes of human enterocytes into M cells that transport bacteria. Science 1997, 277:949–952.
Disclosure
No potential conflict of interest relevant to this article was reported.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pickard, J.M., Chervonsky, A.V. Sampling of the Intestinal Microbiota by Epithelial M Cells. Curr Gastroenterol Rep 12, 331–339 (2010). https://doi.org/10.1007/s11894-010-0128-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11894-010-0128-x