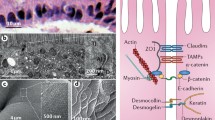
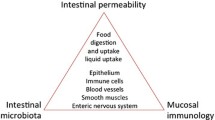
Abstract
This article discusses the concept of intestinal permeability and the barrier function of the gut, elaborates on tight junction structure and the dynamic nature of its composition, outlines the methods for evaluating intestinal permeability, and explores abnormal intestinal permeability in clinical disease, emphasizing its possible role in the pathogenesis of autoimmune conditions. Evidence is provided from several representative diseases for a proposed model of abnormal intestinal permeability in autoimmune disease, including a description of a molecular pathway involving a signaling protein called zonulin, which appears to regulate intestinal permeability. Finally, we speculate on mechanisms that may be responsible for increasing intestinal permeability and consider clinical implications.
Similar content being viewed by others
References and Recommended Reading
Claude P, Goodenough DA: Fracture faces of zonulae occludentes from tight and leaky epithelia. J Cell Biol 1973, 58:390–400.
Cereijido M, Contreras RG, Flores-Benitez D, et al.: New diseases derived or associated with the tight junction. Arch Med Res 2007, 38:465–478.
Koval M: Claudins—key pieces in the tight junction puzzle. Cell Commun Adhes 2006, 13: 127–138.
Itoh M, Furuse M, Morita K, et al.: Direct binding of three tight junction-associated MAGUKs, ZO-1, ZO-2, and ZO-3, with the COOH termini of claudins. J Cell Biol 1999, 147:1351–1363.
Saitou M, Furuse M, Sasaki H, et al.: Complex phenotype of mice lacking occludin, a component of tight junction strands. Mol Biol Cell 2000, 11:4131–4142.
Arrieta MC, Bistritz L, Meddings JB: Alterations in intestinal permeability. Gut 2006, 55:1512–1520.
Shen L, Turner JR: Role of epithelial cells in initiation and propagation of intestinal inflammation. Eliminating the static: tight junction dynamics exposed. Am J Physiol Gastrointest Liver Physiol 2006, 290:G577–G582.
Fujita K, Katahira J, Horiguchi Y, et al.: Clostridium perfringens enterotoxin binds to the second extracellular loop of claudin-3, a tight junction integral membrane protein. FEBS Lett 2000, 476:258–261.
Wang W, Uzzau S, Goldblum SE, et al.: Human zonulin, a potential modulator of intestinal tight junctions. J Cell Sci 2000, 113:4435–4440.
Bjarnason I, Macpherson A, Hollander D: Intestinal permeability: an overview. Gastroenterology 1995, 108:1566–1581.
Meddings JB, Sutherland LR, Byles NI, et al.: Sucrose: a novel permeability marker for gastroduodenal disease. Gastroenterology 1993, 104:1619–1626.
Meddings JB, Gibbons I: Discrimination of site-specific alterations in gastrointestinal permeability in the rat. Gastroenterology 1998, 114:83–92.
Fihn BM, Sjoqvist A, Jodal M: Permeability of the rat small intestinal epithelium along the villus-crypt axis: effects of glucose transport. Gastroenterology 2000, 119:1029–1036.
Scott FW: Food-induced type 1 diabetes in the BB rat. Diabetes Metab Rev 1996, 12:341–359.
Meddings JB, Jarand J, Urbanski SJ, et al.: Increased gastrointestinal permeability as an early lesion in the spontaneously diabetic BB rat. Am J Physiol 1999, 276:G951–G957.
Watts T, Berti I, Sapone A, et al.: Role of the intestinal tight junction modulator zonulin in the pathogenesis of type 1 diabetes in BB diabetic-prone rats. Proc Natl Acad Sci U S A 2005, 102:2916–2921.
Clemente MG, De Virgilis S, Kang JS, et al.: Early effects of gliadin on enterocyte intracellular signaling involved in intestinal barrier function. Gut 2003, 52:218–223.
Barbeau WE, Bassaganya-Riera J, Hontecillas R: Putting the pieces of the puzzle together—a series of hypotheses on the etiology and pathogenesis of type 1 diabetes. Med Hypotheses 2007, 68:607–619.
Secondulfo M, Iafusco D, Carratu R, et al.: Ultrastructural mucosal alterations and increased permeability in non-celiac, type 1 diabetic patients. Dig Liver Dis 2004, 36:35–45.
Sapone A, De Magistris L, Pietzak M, et al.: Zonulin upregulation is associated with increased gut permeability in subjects with type 1 diabetes and their relatives. Diabetes 2006, 55:1443–1449.
Bosi E, Molteni L, Radaelli MG, et al.: Increased intestinal permeability precedes clinical onset of type 1 diabetes. Diabetologia 2006, 49:2824–2827.
Wyatt J, Oberhuber G, Pongratz S, et al.: Increased gastric and intestinal permeability in patients with Crohn’s disease. Am J Gastroenterology 1997, 92:1891–1896.
Sanderson IR, Boulton P, Menzies I, et al.: Improvement of abnormal lactulose/rhamnose permeability in active Crohn’s disease of the small bowel by an elemental diet. Gut 1987, 28:1073–1076.
Wyatt J, Vogelsang H, Hubl W, et al.: Intestinal permeability and the prediction of relapse in Crohn’s disease. Lancet 1993, 341:1437–1439.
Miele E, Pascarella F, Quaglietta L, et al.: Altered intestinal permeability is predictive of early relapse in children with steroid-responsive ulcerative colitis. Aliment Pharmacol Ther 2007, 25:933–939.
Hollander D, Vadheim CM, Brettholz E, et al.: Increased intestinal permeability in patients with Crohn’s disease and their relatives. A possible etiologic factor. Ann Intern Med 1986, 105:883–885.
May GR, Sutherland LR, Meddings JB: Is small intestinal permeability really increased in relatives of patients with Crohn’s disease? Gastroenterology 1993, 104:1627–1632.
D’Inca R, Annese V, di Leo V, et al.: Increased intestinal permeability and NOD2 variants in familial and sporadic Crohn’s disease. Aliment Pharmacol Ther 2006, 23:1455–1461.
Buhner S, Buning C, Genschel J, et al.: Genetic basis for increased intestinal permeability in families with Crohn’s disease: role of CARD15 3020insC mutation? Gut 2006, 55:342–347.
Heller F, Florian P, Bojarski C, et al.: Interleukin-13 is the key effector th2 cytokine in ulcerative colitis that affects epithelial tight junctions, apoptosis, and cell restitution. Gastroenterology 2005, 129:550–564.
Amasheh S, Meiri N, Gitter AH, et al.: Claudin-2 expression induces cation-selective channels in tight junctions of epithelial cells. J Cell Sci 2002, 115:4969–4976.
Poritz LS, Garver KI, Green C, et al.: Loss of the tight junction protein ZO-1 in dextran sulfate sodium induced colitis. J Surg Res 2007, 140:12–19.
Zeissig S, Burgel N, Gunzel D, et al.: Changes in expression and distribution of claudin 2, 5 and 8 lead to discontinuous tight junctions and barrier dysfunction in active Crohn’s disease. Gut 2007, 56:61–72.
Van Heel DA, Hunt K, Greco L, et al.: Genetics in celiac disease. Best Pract Res Clin Gastroenterol 2005, 19:323–329.
Matysiak-Budnik T, Candalh C, Dugave C, et al.: Alterations of the intestinal transport and processing of gliadin peptides in celiac disease. Gastroenterology 2003, 125:696–707.
Fasano A, Not T, Wang W, et al.: Zonulin, a newly discovered modulator of intestinal permeability, and its expression in celiac disease. Lancet 2000, 355:1518–1519.
Duerksen DR, Wilhelm-Boyles C, Parry DM: Intestinal permeability in long-term follow-up of patients with celiac disease on a gluten-free diet. Dig Dis Sci 2005, 50:785–790.
Vilela EG, de Abreu Ferrari ML, de Gama Torres HO, et al.: Intestinal permeability and antigliadin antibody test for monitoring adult patients with celiac disease. Dig Dis Sci 2007, 52:1304–1309.
Hall EJ, Batt RM: Abnormal permeability precedes the development of a gluten sensitive enteropathy in Irish setter dogs. Gut 1991, 32:749–753.
Smecuol E, Sugai E, Niveloni S, et al.: Permeability, zonulin production, and enteropathy in dermatitis herpetiformis. Clin Gastroenterol Hepatol 2005, 3:335–341.
Thomas KE, Sapone A, Fasano A, et al.: Gliadin stimulation of murine macrophage inflammatory gene expression and intestinal permeability are MyD88-dependent: role of the innate immune response in celiac disease. J Immunol 2006, 176:2512–2521.
Drago S, El Asmar R, Di Pierro M, et al.: Gliadin, zonulin and gut permeability: effects on celiac and non-celiac intestinal mucosa and intestinal cell lines. Scand J Gastroenterol 2006, 41:408–419.
Kiesslich R, Goetz M, Angus EA, et al.: Identification of epithelial gaps in human small and large intestine by confocal endomicroscopy. Gastroenterology 2007, 133:1769–1778.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Teshima, C.W., Meddings, J.B. The measurement and clinical significance of intestinal permeability. Curr Gastroenterol Rep 10, 443–449 (2008). https://doi.org/10.1007/s11894-008-0083-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11894-008-0083-y