Abstract
Purpose of Review
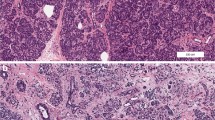
Type 1 diabetes (T1D) is one of the most frequent chronic autoimmune diseases in humans, characterized by the lack of insulin production resulting in high blood glucose levels and lifelong requirement of exogenous insulin administration for survival. It is now recognized that the autoimmune process begins years before the clinical onset, in a stage called pre-symptomatic T1D, in which the presence of β-cell-specific autoantibodies is detectable. Our aim is to review evidence for T1D as a “whole-pancreas disease,” featured by both endocrine and exocrine pancreas alterations already at early disease stages.
Recent Findings
In this review, we discuss a series of recent observations indicating that in genetically predisposed individuals, structural and functional abnormalities as well as immune cell infiltration of the exocrine pancreas are already present in the pre-symptomatic stages of the disease.
Summary
Despite T1D being considered a β-cell-specific disease, numerous reports point to the presence of exocrine pancreas subclinical abnormalities occurring during disease development. These observations challenge the long-standing idea that T1D exocrine damage exists as a mere consequence of disease progression and provide further explanation of mechanisms underlying T1D pathogenesis.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Pollard HM, Miller L, Brewer WA. The external secretion of the pancreas and diabetes mellitus. Am J Dig Dis. 1943;10:20–3.
Gepts W. Pathologic anatomy of the pancreas in juvenile diabetes mellitus. Diabetes. 1965;14:619–33.
Tan GD. The pancreas. Anaesth Intensive Care Med. 2008;9:424–7.
Mohapatra S, Majumder S, Smyrk TC, Zhang L, Matveyenko A, Kudva YC, et al. Diabetes mellitus is associated with an exocrine pancreatopathy: conclusions from a review of literature. Pancreas. 2016;45:1104–10.
Campbell-Thompson M, Rodriguez-Calvo T, Battaglia M. Abnormalities of the exocrine pancreas in type 1 diabetes. Curr Diab Rep. 2015;15:79.
Rewers M, Ludvigsson J. Environmental risk factors for type 1 diabetes. Lancet. 2016;387:2340–8.
DiMeglio LA, Evans-Molina C, Oram RA. Type 1 diabetes. Lancet. 2018;391:2449–62.
Barrett JC, Clayton DG, Concannon P, et al. Genome-wide association study and meta-analysis find that over 40 loci affect risk of type 1 diabetes. Nat Genet. 2009;41:703–7.
Raeder H, Johansson S, Holm PI, et al. Mutations in the CEL VNTR cause a syndrome of diabetes and pancreatic exocrine dysfunction. Nat Genet. 2006;38:54–62.
Panicot L, Mas E, Thivolet C, Lombardo D. Circulating antibodies against an exocrine pancreatic enzyme in type 1 diabetes. Diabetes. 1999;48:2316–23.
Endo T, Takizawa S, Tanaka S, Takahashi M, Fujii H, Kamisawa T, et al. Amylase alpha-2A autoantibodies: novel marker of autoimmune pancreatitis and fulminant type 1 diabetes. Diabetes. 2009;58:732–7.
Taniguchi T, Okazaki K, Okamoto M, Seko S, Tanaka J, Uchida K, et al. High prevalence of autoantibodies against carbonic anhydrase II and lactoferrin in type 1 diabetes: concept of autoimmune exocrinopathy and endocrinopathy of the pancreas. Pancreas. 2003;27:26–30.
Kobayashi T, Nakanishi K, Kajio H, Morinaga S, Sugimoto T, Murase T, et al. Pancreatic cytokeratin: an antigen of pancreatic exocrine cell autoantibodies in type 1 (insulin-dependent) diabetes mellitus. Diabetologia. 1990;33:363–70.
Dooley J, Tian L, Schonefeldt S, Delghingaro-Augusto V, Garcia-Perez JE, Pasciuto E, et al. Genetic predisposition for beta cell fragility underlies type 1 and type 2 diabetes. Nat Genet. 2016;48:519–27.
Ashrafi G, Schwarz TL. The pathways of mitophagy for quality control and clearance of mitochondria. Cell Death Differ. 2013;20:31–42.
Hakonarson H, Grant SFA, Bradfield JP, Marchand L, Kim CE, Glessner JT, et al. A genome-wide association study identifies KIAA0350 as a type 1 diabetes gene. Nature. 2007;448:591–4.
Williams AJK, Thrower SL, Sequeiros IM, Ward A, Bickerton AS, Triay JM, et al. Pancreatic volume is reduced in adult patients with recently diagnosed type 1 diabetes. J Clin Endocrinol Metab. 2012;97:E2109–13.
Campbell-Thompson M, Wasserfall C, Montgomery EL Pancreas organ weight in individuals with disease-associated autoantibodies at risk for type 1 diabetes. jamanetwork.com
Foulis AK, Stewart JA. The pancreas in recent-onset type 1 (insulin-dependent) diabetes mellitus: insulin content of islets, insulitis and associated changes in the exocrine acinar tissue. Diabetologia. 1984;26:456–61.
Rahier J, Goebbels RM, Henquin JC. Cellular composition of the human diabetic pancreas. Diabetologia. 1983;24:366–71.
Virostko J, Hilmes M, Eitel K, Moore DJ, Powers AC. Use of the electronic medical record to assess pancreas size in type 1 diabetes. PLoS One. 2016;11:e0158825.
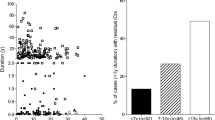
•• Virostko J, Williams J, Hilmes M, Bowman C, Wright JJ, Du L, et al. Pancreas volume declines during the first year after diagnosis of type 1 diabetes and exhibits altered diffusion at disease onset. Diabetes Care. 2019;42:248–57. This is an important study describing not only a reduction in terms of pancreatic volume but also a change in pancreas composition in pre-symptomatic and symptomatic T1D with the use of modern technologies.
•• Campbell-Thompson M, Atkinson MA, Filipp SL, Gurka MJ, Beegle R, Schatz D, Haller MJ, TYPE 1 DIABETES STUDY GROUP (2018) Relative pancreas volume is reduced in autoantibody negative first-degree relatives and subjects with pre–type 1 diabetes. Diabetes 67:1816–P. This is first demonstration of volume reduction in the very early phases of T1D development with the use of MRI.
Gaglia JL, Guimaraes AR, Harisinghani M, Turvey SE, Jackson R, Benoist C, et al. Noninvasive imaging of pancreatic islet inflammation in type 1A diabetes patients. J Clin Invest. 2011;121:442–5.
Gilbeau JP, Poncelet V, Libon E, Derue G, Heller FR. The density, contour, and thickness of the pancreas in diabetics: CT findings in 57 patients. AJR Am J Roentgenol. 1992;159:527–31.
Sequeiros IM, Hester K, Callaway M, Williams A, Garland Z, Powell T, et al. MRI appearance of the pancreas in patients with cystic fibrosis: a comparison of pancreas volume in diabetic and non-diabetic patients. Br J Radiol. 2010;83:921–6.
Henderson JR, Daniel PM, Fraser PA. The pancreas as a single organ: the influence of the endocrine upon the exocrine part of the gland. Gut. 1981;22:158–67.
Bonnet-Serrano F, Diedisheim M, Mallone R, Larger E. Decreased α-cell mass and early structural alterations of the exocrine pancreas in patients with type 1 diabetes: an analysis based on the nPOD repository. PLoS One. 2018;13:e0191528.
Whitcomb DC, Lowe ME. Human pancreatic digestive enzymes. Dig Dis Sci. 2007;52:1–17.
Vacca JB, Henke WJ, Knight WA. The exocrine pancreas in diabetes mellitus. Ann Intern Med. 1964;61:242–7.
Lankisch PG, Manthey G, Otto J, Koop H, Talaulicar M, Willms B, et al. Exocrine pancreatic function in insulin-dependent diabetes mellitus. Digestion. 1982;25:211–6.
Domínguez-Muñoz JE, Hieronymus C, Sauerbruch T, Malfertheiner P. Fecal elastase test: evaluation of a new noninvasive pancreatic function test. Am J Gastroenterol. 1995;90:1834–7.
Hardt PD, Krauss A, Bretz L, Porsch-Ozcürümez M, Schnell-Kretschmer H, Mäser E, et al. Pancreatic exocrine function in patients with type 1 and type 2 diabetes mellitus. Acta Diabetol. 2000;37:105–10.
Hardt PD, Hauenschild A, Nalop J, Marzeion AM, Jaeger C, Teichmann J, et al. High prevalence of exocrine pancreatic insufficiency in diabetes mellitus. A multicenter study screening fecal elastase 1 concentrations in 1,021 diabetic patients. Pancreatology. 2003;3:395–402.
Larger E, Philippe MF, Barbot-Trystram L, Radu A, Rotariu M, Nobécourt E, et al. Pancreatic exocrine function in patients with diabetes. Diabet Med. 2012;29:1047–54.
Chen J-M, Radisky ES, Férec C (2013) Human trypsins. Handbook of proteolytic enzymes. Elsevier, pp 2600–2609.
•• Li X, Campbell-Thompson M, Wasserfall CH, et al. Serum trypsinogen levels in type 1 diabetes. Diabetes Care. 2017;40:577–82. The first paper reporting a decreased level of pancreatic enzymes already in pre-symptomatic T1D.
Oh H-C, Kwon C-I, El Hajj II, Easler JJ, Watkins J, Fogel EL, et al. Low serum pancreatic amylase and lipase values are simple and useful predictors to diagnose chronic pancreatitis. Gut Liver. 2017;11:878–83.
Treacy J, Williams A, Bais R, Willson K, Worthley C, Reece J, et al. Evaluation of amylase and lipase in the diagnosis of acute pancreatitis. ANZ J Surg. 2001;71:577–82.
Moossa AR. Current concepts. Diagnostic tests and procedures in acute pancreatitis. N Engl J Med. 1984;311:639–43.
• Lundberg M, Lindqvist A, Wierup N, Krogvold L, Dahl-Jørgensen K, Skog O. The density of parasympathetic axons is reduced in the exocrine pancreas of individuals recently diagnosed with type 1 diabetes. PLoS One. 2017;12:e0179911. The first evidence that the density of parasympathetic axons innervating the exocrine pancreas is decreased in patients with recent T1D onset.
Stamatouli AM, Quandt Z, Perdigoto AL, Clark PL, Kluger H, Weiss SA, et al. Collateral damage: insulin-dependent diabetes induced with checkpoint inhibitors. Diabetes. 2018;67:1471–80.
Obata A, Kaneto H, Kamei S, Shimoda M, Kishi S, Isogawa A, et al. Pancreatic inflammation captured by imaging technology at the onset of fulminant type 1 diabetes: figure 1. Diabetes Care. 2015;38:e135–6.
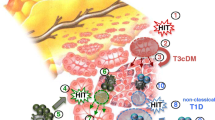
Rodriguez-Calvo T, Ekwall O, Amirian N, Zapardiel-Gonzalo J, von Herrath MG. Increased immune cell infiltration of the exocrine pancreas: a possible contribution to the pathogenesis of type 1 diabetes. Diabetes. 2014;63:3880–90.
Vecchio F, Lo Buono N, Stabilini A, Nigi L, Dufort MJ, Geyer S, et al. Abnormal neutrophil signature in the blood and pancreas of presymptomatic and symptomatic type 1 diabetes. JCI Insight. 2018. https://doi.org/10.1172/jci.insight.122146.
Valle A, Giamporcaro GM, Scavini M, Stabilini A, Grogan P, Bianconi E, et al. Reduction of circulating neutrophils precedes and accompanies type 1 diabetes. Diabetes. 2013;62:2072–7.
Merza M, Hartman H, Rahman M, Hwaiz R, Zhang E, Renström E, et al. Neutrophil extracellular traps induce trypsin activation, inflammation, and tissue damage in mice with severe acute pancreatitis. Gastroenterology. 2015;149:1920–1931.e8.
Leppkes M, Maueröder C, Hirth S, Nowecki S, Günther C, Billmeier U, et al. Externalized decondensed neutrophil chromatin occludes pancreatic ducts and drives pancreatitis. Nat Commun. 2016;7:10973.
Diana J, Simoni Y, Furio L, Beaudoin L, Agerberth B, Barrat F, et al. Crosstalk between neutrophils, B-1a cells and plasmacytoid dendritic cells initiates autoimmune diabetes. Nat Med. 2013;19:65–73.
Harsunen MH, Puff R, D’Orlando O, Giannopoulou E, Lachmann L, Beyerlein A, et al. Reduced blood leukocyte and neutrophil numbers in the pathogenesis of type 1 diabetes. Horm Metab Res. 2013;45:467–70.
Qin J, Fu S, Speake C, Greenbaum CJ, Odegard JM. NETosis-associated serum biomarkers are reduced in type 1 diabetes in association with neutrophil count. Clin Exp Immunol. 2016;184:318–22.
Wang Y, Xiao Y, Zhong L, Ye D, Zhang J, Tu Y, et al. Increased neutrophil elastase and proteinase 3 and augmented NETosis are closely associated with β-cell autoimmunity in patients with type 1 diabetes. Diabetes. 2014;63:4239–48.
Bollyky JB, Xu P, Butte AJ, Wilson DM, Beam CA, Greenbaum CJ, et al. Heterogeneity in recent-onset type 1 diabetes - a clinical trial perspective. Diabetes Metab Res Rev. 2015;31:588–94.
Newby BN, Mathews CE. Type I interferon is a catastrophic feature of the diabetic islet microenvironment. Front Endocrinol (Lausanne). 2017;8:232.
Huang X, Yuang J, Goddard A, Foulis A, James RF, Lernmark A, et al. Interferon expression in the pancreases of patients with type I diabetes. Diabetes. 1995;44:658–64.
Nejentsev S, Walker N, Riches D, Egholm M, Todd JA. Rare variants of IFIH1, a gene implicated in antiviral responses, protect against type 1 diabetes. Science. 2009;324:387–9.
Foulis AK, Farquharson MA, Meager A. Immunoreactive alpha-interferon in insulin-secreting beta cells in type 1 diabetes mellitus. Lancet. 1987;2:1423–7.
•• Damond N, Engler S, Zanotelli VRT, et al. A map of human type 1 diabetes progression by imaging mass cytometry. Cell Metab. 2019;29:755–768.e5. This is a comprehensive mapping of immune cell localization in the pancreas from patients with T1D using a novel technology called imaging mass cytometry.
In’t Veld P. Insulitis in human type 1 diabetes: the quest for an elusive lesion. Islets. 2011;3:131–8.
Ernst D, Witte T. Sjögren’s syndrome. Dtsch Med Wochenschr. 2016;141:544–50.
Nordmark G, Eloranta M-L, Ronnblom L. Primary Sjögren’s syndrome and the type I interferon system. Curr Pharm Biotechnol. 2012;13:2054–62.
Nezos A, Gravani F, Tassidou A, Kapsogeorgou EK, Voulgarelis M, Koutsilieris M, et al. Type I and II interferon signatures in Sjogren’s syndrome pathogenesis: contributions in distinct clinical phenotypes and Sjogren’s related lymphomagenesis. J Autoimmun. 2015;63:47–58.
Soza A, Everhart JE, Ghany MG, Doo E, Heller T, Promrat K, et al. Neutropenia during combination therapy of interferon alfa and ribavirin for chronic hepatitis C. Hepatology. 2002;36:1273–9.
de Bruin AM, Libregts SF, Valkhof M, Boon L, Touw IP, Nolte MA. IFNγ induces monopoiesis and inhibits neutrophil development during inflammation. Blood. 2012;119:1543–54.
Manns MP, McHutchison JG, Gordon SC, Rustgi VK, Shiffman M, Reindollar R, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358:958–65.
TEDDY Study Group. The environmental determinants of diabetes in the young (TEDDY) study. Ann N Y Acad Sci. 2008;1150:1–13.
Battaglia M, Anderson MS, Buckner JH, Geyer SM, Gottlieb PA, Kay TWH, et al. Understanding and preventing type 1 diabetes through the unique working model of TrialNet. Diabetologia. 2017;60:2139–47.
Acknowledgments
We acknowledge Manuela Battaglia for her valuable and constructive suggestions during the development of this work.
Funding
Part of the work here described was generated thanks to the support of the Juvenile Diabetes Research Foundation (#3-SRA-2016-262-S-B) and Fondazione Cariplo (grant no. 2013-0941). Alessandra Petrelli is supported by the European Commission (grant no. H2020-MSCA-IF-2015 - 704779) and the Axa Research Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Pathogenesis of Type 1 Diabetes
Rights and permissions
About this article
Cite this article
Vecchio, F., Messina, G., Giovenzana, A. et al. New Evidence of Exocrine Pancreatopathy in Pre-symptomatic and Symptomatic Type 1 Diabetes. Curr Diab Rep 19, 92 (2019). https://doi.org/10.1007/s11892-019-1223-5
Published:
DOI: https://doi.org/10.1007/s11892-019-1223-5