Abstract
Purpose of Review
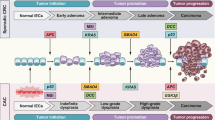
Colorectal cancer (CRC) is the fourth most common cancer in both men and women in the USA, resulting in over 55,000 deaths annually. Environmental and genetic factors influence the development of CRC, and inflammation is a critical hallmark of cancer that may arise from a variety of factors. While patients with inflammatory bowel disease (IBD) have a higher risk of developing CRC, sporadic CRCs may engender or be potentiated by inflammation as well. In this review, we focus on recent advances in basic and translational research utilizing murine models to understand the contribution of inflammatory signaling pathways to CRC.
Recent Findings
We discuss advances in the utility of three-dimensional enteroid/colonoid/tumoroid cultures to understand immune-epithelial interactions in CRC, as well as the potential for utilizing patient-derived tumoroids for personalized therapies.
Summary
This review underscores the importance of understanding the complex molecular mechanisms underlying inflammation in sporadic CRC and highlights up-and-coming or new avenues for CRC biomarkers or therapies.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Howlader N, Noone AM, Krapcho M et al. SEER Cancer Statistics Review, 1975–2013. Bethesda: Natl. Cancer Institute; [Internet] 2016. Available from: http://seer.cancer.gov/csr/1975_2013/.
Dulai PS, Sandborn WJ, Gupta S. Colorectal cancer and dysplasia in inflammatory bowel disease: a review of disease epidemiology, pathophysiology, and management. Cancer Prev Res [Internet]. 2016;9:887–94. Available from: http://cancerpreventionresearch.aacrjournals.org/content/early/2016/11/07/1940-6207.CAPR-16-0124.abstract
Fearon EF, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61:759–67.
Kern SE, Redston M, Seymour AB, Caldas C, Powell SM, Kornacki S, et al. Molecular genetic profiles of colitis-associated neoplasms. Gastroenterology. 1994;107:420–8.
Friis S, Riis AH, Erichsen R, Baron JA, Sorensen HT. Low-dose aspirin or nonsteroidal anti-inflammatory drug use and colorectal cancer risk: a population-based, case-control study. Ann Intern Med. 2015;163:347–55.
Graham DM, Coyle VM, Kennedy RD, Wilson RH. Molecular subtypes and personalized therapy in metastatic colorectal cancer. Curr Colorectal Cancer Rep. 2016;12:141–50.
•• Guinney J, Dienstmann R, Wang X, de Reyniès A, Schlicker A, Soneson C, et al. The consensus molecular subtypes of colorectal cancer. Nat Med. 2015;21:1350–6. Describes novel classification system for CRC via coalescence of six independent CRC classification systems into four consensus molecular subtypes (CMS).
• Müller MF, Ibrahim AEK, Arends MJ. Molecular pathological classification of colorectal cancer. Virchows Arch. 2016;469:125–34. Evaluates molecular changes in genetic instability in CRC and describes majority of hypermutated microsatellite instability (MSI) cancers falling into consensus molecular subtype 1 (CMS1, MSI-immune).
Becht E, De Reyniies A, Giraldo NA, Pilati C, Buttard B, Lacroix L, et al. Immune and stromal classification of colorectal cancer is associated with molecular subtypes and relevant for precision immunotherapy. Clin Cancer Res. 2016;22:4057–66.
Terzić J, Grivennikov S, Karin E, Karin M. Inflammation and colon cancer. Gastroenterology 2010;138:2101–14.
Calle EE. Obesity and cancer. Br Med J. 2007;335:1107–8.
Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pagès C, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–4.
Lasry A, Zinger A, Ben-neriah Y. Review Inflammatory networks underlying colorectal cancer. Nat Immunol. 2016;17:230–40.
•• Grivennikov SI, Wang K, Mucida D, Stewart CA, Schnabl B, Jauch D, et al. Adenoma-linked barrier defects and microbial products drive IL-23/IL-17-mediated tumour growth. Nature. 2012;491:254–8. Demonstrates role of IL-23 in eliciting Th17 pro-tumoriencic responses.
Okayasu I, Hatakeyama S, Yamada M, Ohkusa T, Inagaki Y, Nakaya R. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology. 1990;98:694–702.
Cooper HS, Murthy SN, Shah RS, Sedergran DJ. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Investig. 1993;69:238–49.
Tanaka T, Kohno H, Suzuki R, Yamada Y, Sugie S, Mori H. A novel inflammation-related mouse colon carcinogenesis model induced by azoxymethane and dextran sodium sulfate. Cancer Sci. 2003;94:965–73.
Maloy KJ, Salaun L, Cahill R, Dougan G, Saunders NJ, Powrie F. CD4 + CD25 + T R cells suppress innate immune pathology through cytokine-dependent mechanisms. J Exp Med. 2003;197:111–9.
Kirchberger S, Royston DJ, Boulard O, Thornton E, Franchini F, Szabady RL, et al. Innate lymphoid cells sustain colon cancer through production of interleukin-22 in a mouse model. J Exp Med. 2013;210:917–31.
Berg DJ, Davidson N, Kühn R, Müller W, Menon S, Holland G, et al. Enterocolitis and colon cancer in interleukin-10-deficient mice are associated with aberrant cytokine production and CD4 ϩ TH1-like responses. J Clin Invest. 1996;98(4):1010–20.
Davidson NJ, Leach MW, Fort MM, Thompson-snipes L, Kiihnfl R, Mi W, et al. T helper cell 1-type C D 4 + T cells, but not B cells mediate colitis in interleukin 10-deficient mice. J Exp Med 1996;184:241–51.
Su L, Kinzler KW, Vogelstein B, Preisinger AC, Moser R, Luongo C, et al. Multiple intestinal neoplasia caused by a mutation in the murine homolog of the APC Gene Published by: American Association for the Advancement of Science Stable URL: http://www.jstor.org/stable/2876870 Multiple intestinal neoplasia caused by a mutation. 1992;256:668–70.
Gounari F, Chang R, Cowan J, Guo Z, Dose M, Gounaris E, et al. Loss of adenomatous polyposis coli gene function disrupts thymic development. Nat Immunol. 2005;6:800–9.
Cheung AF, Carter AM, Kostova KK, Woodruff JF, Crowley D, Bronson RT, et al. Complete deletion of Apc results in severe polyposis in mice. Oncogene. 2010;29:1857–64.
Smits R, Van Oordt WVH, Luz A, Zurcher C, Jagmohan-Changur S, Breukel C, et al. Apc1638N: a mouse model for familial adenomatous polyposis-associated desmoid tumors and cutaneous cysts. Gastroenterology. 1998;114:275–83.
Hinoi T, Akyol A, Theisen BK, Ferguson DO, Greenson JK, Williams BO, et al. Mouse model of colonic adenoma-carcinoma progression based on somatic Apc inactivation. Cancer Res. 2007;67:9721–30.
Chaudhry A, Samstein RM, Treuting P, Liang Y, Pils MC, Heinrich J, et al. Interleukin-10 signaling in regulatory T cells is required for suppression of Th17 cell-mediated inflammation. Immunity. 2011;34(4):566–78.
Dennis KL, Saadalla A, Blatner NR, Wang S, Venkateswaran V, Gounari F, et al. T-cell expression of IL10 is essential for tumor immune surveillance in the small intestine. Cancer Immunol Res. 2015;3:806–15.
•• Dennis KL, Wang Y, Blatner NR, Wang S, Saadalla A, Trudeau E, et al. Adenomatous polyps are driven by microbe-instigated focal in flammation and are controlled by IL-10-producing T cells. Cancer Res. 2013;73(19):5905–13. Described influence of microbiota on eliciting IL-10 response and requirement of IL-10 to donwregulate tumorigenesis.
Wang L, Wang Y, Song Z, Chu J, Qu X. Deficiency of interferon-gamma or its receptor promotes colorectal cancer development. J Interf Cytokine Res. 2015;35:273–80.
Tjandra SS, Hsu C, Goh I, Gurung A, Poon R, Nadesan P, et al. IFN-Beta signaling positively regulates tumorigenesis in aggressive fibromatosis, potentially by modulating mesenchymal progenitors. Cancer Res. 2007;67:7124–31.
•• Katlinski KV, Gui J, Katlinskaya YV, Koumenis C, Rui H, Fuchs SY. Inactivation of interferon receptor promotes the establishment of immune privileged tumor article inactivation of interferon receptor promotes the establishment of immune privileged tumor microenvironment. Cancer Cell. 2017;31:194–207. Demonstrates that tumor cells can downregulate type I interferon receptors to reduce effectiveness of PD-1 blockade. Stabilization of INFRa decreases tumor size and incidence.
Song X, Gao H, Lin Y, Yao Y, Zhu S, Wang J, et al. Alterations in the microbiota drive interleukin-17C production from intestinal epithelial cells to promote tumorigenesis. Immunity. 2013;40:140–52.
Richter C, Herrero San Juan M, Weigmann B, Bergis D, Dauber K, Muders MH, et al. Defective IL-23/IL-17 Axis protects p47phox−/− mice from colon cancer. Front Immunol. 2017;8:1–10.
Popivanova BK, Kitamura K, Wu Y, Kondo T, Kagaya T, Kaneko S, et al. Blocking TNF-α in mice reduces colorectal carcinogenesis associated with chronic colitis. J Clin Invest. 2008;118:560–70.
Hale LP, Greer PK. A novel murine model of inflammatory bowel disease and inflammation-associated Colon cancer with ulcerative colitis-like features. PLoS One. 2012;7:e41797.
Blatner NR, Mulcahy MF, Dennis KL, Scholtens D, Bentrem DJ, Phillips JD, et al. Expression of RORγt marks a pathogenic regulatory T cell subset in human colon cancer. Sci Trans Med. 2012;4:164ra159.
Greten FR, Eckmann L, Greten TF, Park JM, Li Z, Egan LJ, et al. IKK-beta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell. 2004;118:285–96.
Shaked H, Hofseth LJ, Chumanevich A, Chumanevich AA, Wang J, Wang Y. Chronic epithelial NF-κB activation accelerates APC loss and intestinal tumor initiation through iNOS up-regulation. Proc Natl Acad Sci. 2012;109:14007–12.
Koliaraki V, Pasparakis M, Kollias G. IKKβ in intestinal mesenchymal cells promotes initiation of colitis-associated cancer. J Exp Med. 2015;212:2235–51.
Grivennikov S, Karin E, Terzic J, Mucida D, Yu G, Vallabhapurapu S, et al. IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell. 2009;15:103–13.
Baltgalvis KA, Berger FG, Pena MMO, Davis JM, Muga SJ, Carson JA, et al. Interleukin-6 and cachexia in Apc min /+ mice. Am J Physiol Regul Integr Comp Physiol. 2008;294:393–401.
Bollrath J, Phesse TJ, Von Burstin VA, Putoczki T, Bennecke M, Bateman T, et al. Article gp130-mediated Stat3 activation in enterocytes regulates cell survival and cell-cycle progression during colitis-associated tumorigenesis. Cancer Cell. 2009;15:91–102.
Musteanu M, Blaas L, Mair M, Schlederer M, Bilban M, Tauber S, et al. Stat3 is a negative regulator of intestinal tumor progression in ApcMin mice. Gastroenterology. 2010;138:1003–1011.e5.
Pathria P, Gotthardt D, Prchal-Murphy M, Putz E-M, Holcmann M, Schlederer M, et al. Myeloid STAT3 promotes formation of colitis-associated colorectal cancer in mice. Oncoimmunology. 2015;4:e998529.
Putoczki TL, Thiem S, Loving A, Busuttil RA, Wilson NJ, Ziegler PK, et al. Interleukin-11 is the dominant IL-6 family cytokine during Gastrointestinal tumorigenesis and can be targeted therapeutically. Cancer Cell. 2013;24:257–71.
Oshima M, Dinchuk JE, Kargman SL, Oshima H, Hancock B, Kwong E, et al. Suppression of intestinal polyposis in Apc-delta 716 knockout mice by inhibition of cyclooxygenase 2 ( COX-2). Cell. 1996;87:803–9.
Chulada PC, Thompson MB, Mahler JF, Doyle CM, Gaul BW, Lee C, et al. Genetic disruption of Ptgs-1, as well as of Ptgs-2, reduces intestinal tumorigenesis in min mice. Cancer Res. 2000;60:4705–8.
Groden J, Thliveris A, Samowitz W, Carlson M, Gelbert L, Albertsen H, et al. Identification and characterization of the familial adenomatous polyposis coli gene. Cell. 1991;66:589–600.
Polakis P. Wnt signaling in cancer. Cold Spring Harb Perspect Biol. 2012;4(5). doi:10.1101/cshperspect.a008052.
The Cancer Genome Atlas Network. Comprehesive molecular characterization of human colon and rectal cancer. Nature. 2012;487:330–7.
Rapaich A, Pitot HC, Dove WF, Moser AMYR, Pitot HC, Dove WF. A dominant mutation that predisposes to multiple intestinal neoplasia in the mouse published by: American Association for the Advancement of Science Stable URL: http://www.jstor.org/stable/2873632 A dominant mutation that predisposes to multiple intesti. 1990;247:322–4.
Stanilov NS, Miteva L, Cirovski G, Stanilova SA. Increased transforming growth factor β and interleukin 10 transcripts in peripheral blood mononuclear cells of colorectal cancer patients. Contemp Oncol. 2016;20:458–62.
Glocker E-O, Kotlarz D, Boztug K, Gertz EM, Schäffer AA, Noyan F, et al. Inflammatory bowel disease and mutations affecting the interleukin-10 receptor. N Engl J Med. 2009;361:2033–45.
• Chung AY, Li Q, Blair SJ, De Jesus M, Dennis KL, Levea C, et al. Oral interleukin-10 alleviates polyposis via neutralization of pathogenic T-regulatory cells. Cancer Res. 2014;74:5377–85. Showed that oral IL-10 administration can downregulate pathogenic Th17 response in order to reduce tumor size and incidence.
Parker BS, Rautela J, Hertzog PJ. Review. Antitumour actions of interferons: implications for cancer therapy. Nature rev. Cancer. 2016;16:131–44.
McGovern D, Powrie F. The IL23 axis plays a key role in the pathogenesis of IBD. Gut. 2007;56:1333–6.
De Simone V, Pallone F, Monteleone G, De Simone V, Pallone F, Monteleone G, et al. Role of T H 17 cytokines in the control of colorectal cancer. Oncoimmunology. 2013;2(12):e26617.
Tesmer LA, Lundy SK, Sarkar S, Fox DA. Review. Th17 cells in human disease. Immunol Rev. 2008;223:87–113.
Wu P, Wu D, Ni C, Ye J, Chen W, Hu G, et al. ydT17 cells promote the accumulation and expansion of myeloid-derived suppressor cells in human colorectal cancer. Immunity. 2014;40:785–800.
Heikkila K, Ebrahim S, Lawlor DA. Systematic review of the association between circulating interleukin-6 (IL-6) and cancer. Eur J Cancer. 2008;44:937–45.
Olsen J, Kirkeby LT, Olsen J, Eiholm S, Jess PER, Gögenur I, et al. High interleukin-6 mRNA expression is a predictor of relapse in colon cancer. Anticancer Res. 2015;2240:2235–40.
Zhou L, Ivanov II, Spolski R, Min R, Shenderov K, Egawa T, et al. IL-6 programs TH-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol. 2007;8:967–74.
Yu H, Pardoll D, Jove R. Review. STATs in cancer inflammation and immunity: a leading role for STAT3. Nat Rev Cancer. 2009;9:798–809.
Sanchez-Lopez E, Flashner-Abramson E, Shalapour S, Zhong Z, Taniguchi K, Levitzki A, et al. Targeting colorectal cancer via its microenvironment by inhibiting IGF-1 receptor-insulin receptor substrate and STAT3 signaling. Oncogene. 2015;35:1–11.
• Kryczek I, Lin Y, Nagarsheth N, Peng D, Zhao L, Zhao E, et al. Stemness via STAT3 transcription factor activation and induction of the methyltransferase DOT1L. Immunity. 2013;40:772–84. Demonstrates a role for STAT3 signaling, in order to upregulate stemness genes through DOTL1, a methyltransferase.
De Simone V, Franzè E, Ronchetti G, Colantoni A, Fantini MC, Di Fusco D, et al. Th17-type cytokines, IL-6 and TNF-α synergistically activate STAT3 and NF-kB to promote colorectal cancer cell growth. Oncogene. 2015;34:3493–503.
Ben-Neriah Y, Karin M. Review. Inflammation meets cancer, with NF-κB as the matchmaker. Nat. Immunol. 2011;12:715–23.
Nenci A, Becker C, Wullaert A, Gareus R, Van Loo G, Danese S, et al. Epithelial NEMO links innate immunity to chronic intestinal inflammation. Nature. 2007;446:557–61.
• Cooks T, Pateras IS, Tarcic O, Solomon H, Schetter AJ, Wilder S, et al. Mutant p53 prolongs NF-κB activation and promotes chronic inflammation and inflammation-associated colorectal cancer. Cancer Cell. 2013;23:634–46. Demonstrates that in mutant p53 had oncogenic functions in chronic colitis via NF-κB signaling, in order to promote invasive carcinoma.
Oshima H, Hioki K, Popivanova BK, Van Rooijen N, Ishikawa TO, Oshima M. Prostaglandin E2 signaling and bacterial infection recruit tumor-promoting macrophages to mouse gastric tumors. Gastroenterology. 2011;140:596–607.
Zelenay S, Van Der Veen AG, Bottcher JP, Snelgrove KJ, Rogers N, Acton SE, et al. Cyclooxygenase-dependent tumor growth through evasion of immunity. Cell. 2015;162:1257–70.
Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372:2509–20.
Garza-Treviño EN, Said-Fernández SL, Martínez-Rodríguez HG. Understanding the colon cancer stem cells and perspectives on treatment. Cancer Cell Int. 2015;15:2.
Diehn M, Cho RW, Lobo NA, Kalisky T, Jo M, Kulp AN, et al. Association of reactive oxygen species levels and radioresistance in cancer stem cells. Nature. 2009;458:780–3.
O’Brien CA, Kreso A, Ryan P, Hermans KG, Gibson L, Wang Y, et al. ID1 and ID3 regulate the self-renewal capacity of human colon cancer-initiating cells through p21. Cancer Cell. 2012;21:777–92.
Ibrahem S, Al-Ghamdi S, Baloch K, Muhammad B, Fadhil W, Jackson D, et al. STAT3 paradoxically stimulates beta-catenin expression but inhibits beta-catenin function. Int J Exp Pathol. 2014;95:392–400.
Lin L, Liu A, Peng Z, Lin HJ, Li PK, Li C, et al. STAT3 is necessary for proliferation and survival in colon cancer-initiating cells. Cancer Res. 2011;71:7226–37.
Spitzner M, Roesler B, Bielfeld C, Emons G, Gaedcke J, Wolff HA, et al. STAT3 inhibition sensitizes colorectal cancer to chemoradiotherapy in vitro and in vivo. Int J Cancer. 2014;134:997–1007.
Phesse TJ, Buchert M, Stuart E, Flanagan DJ, Faux M, Afshar-Sterle S, et al. Partial inhibition of gp130-Jak-Stat3 signaling prevents Wnt-β-catenin-mediated intestinal tumor growth and regeneration. Sci Signal. 2014;7:ra92.
Lindemans CA, Calafiore M, Mertelsmann AM, O’Connor MH, Dudakov JA, Jenq RR, et al. Interleukin-22 promotes intestinal-stem-cell-mediated epithelial regeneration. Nature. 2015;528:560–4.
Schwitalla S, Fingerle AA, Cammareri P, Nebelsiek T, Göktuna SI, Ziegler PK, et al. Intestinal tumorigenesis initiated by dedifferentiation and acquisition of stem-cell-like properties. Cell. 2013;152:25–38.
Myant KB, Cammareri P, McGhee EJ, Ridgway RA, Huels DJ, Cordero JB, et al. ROS production and NF-kappaB activation triggered by RAC1 facilitate WNT-driven intestinal stem cell proliferation and colorectal cancer initiation. Cell Stem Cell. 2013;12:761–73.
Du Q, Geller DA. Cross-regulation between Wnt and NF-kB signaling pathways. Immunopathol Dis Ther. 2011;1:1–18.
Clevers HC, Bevins CL. Paneth cells: maestros of the small intestinal crypts. Annu Rev Physiol. 2013;75:289–311.
Adolph TE, Tomczak MF, Niederreiter L, Ko H-J, Bock J, Martinez-naves E, et al. Paneth cells as a site of origin for intestinal inflammation. Nature. 2013;503:272–6.
• Feng Y, Sentani K, Wiese A, Sands E, Green M, Bommer GT, et al. Sox9 induction, ectopic paneth cells, and mitotic spindle axis defects in mouse colon adenomatous epithelium arising from conditional biallelic Apc inactivation. Am J Pathol. 2013;183:493–503. Describes ectopic Paneth cell expression driven by Apc inactivation in Cdx2-CreER(T2) model of colon tumorigenesis.
Joo M, Shahsafaei A, Odze RD. Paneth cell differentiation in colonic epithelial neoplasms: evidence for the role of the Apc/beta-catenin/Tcf pathway. Hum Pathol. 2009;40:872–80.
Wada R. Proposal of a new hypothesis on the development of colorectal epithelial neoplasia: nonspecific inflammation-colorectal Paneth cell metaplasia-colorectal epithelial neoplasia. Digestion. 2009;79:9–12.
Wang D, Peregrina K, Dhima E, Lin EY, Mariadason JM, Augenlicht LH. Paneth cell marker expression in intestinal villi and colon crypts characterizes dietary induced risk for mouse sporadic intestinal cancer. Proc Natl Acad Sci. 2011;108:10272–7.
Pai RK, Rybicki LA, Goldblum JR, Shen B, Xiao S-Y, Liu X. Paneth cells in colonic adenomas association with male sex and adenoma burden. Am J Surg Pathol. 2013;37:98–103.
Mahon M, Xu J, Yi X, Liu X, Gao N, Zhang L. Paneth cell in adenomas of the distal colorectum is inversely associated with synchronous advanced adenoma and carcinoma. Sci Rep. 2016;6:26129. doi:10.1038/srep26129.
• Hilkens J, Timmer NC, Boer M, Ikink GJ, Schewe M, Sacchetti A, et al. RSPO3 expands intestinal stem cell and niche compartments and drives tumorigenesis. Gut. 2016;66:1095–105. Describes overexpression of R-spondin 3 (RSPO3) in a subset of CRCs and uses a mouse model of conditional Rspo3 expression driven by Lgr5-GFP-CreER(T2) to demonstrate up-regulation of intestinal stem and Paneth cells as well as adenocarcinoma.
• Nakanishi Y, Reina-Campos M, Nakanishi N, Llado V, Elmen L, Peterson S, et al. Control of Paneth cell fate, intestinal inflammation, and tumorigenesis by PKCλ/Ι. Cell Rep. 2016;16:3297–310. Highlights the role of protein kinase C (PKC) λ/ι in Paneth cell homeostasis, intestinal inflammation, and cancer.
Fujii M, Shimokawa M, Date S, Takano A, Matano M, Nanki K, et al. A colorectal tumor organoid library demonstrates progressive loss of niche factor requirements during tumorigenesis. Cell Stem Cell. 2016;18:827–38.
Young M, Reed KR. Organoids as a model for colorectal cancer. Curr Colorectal Cancer Rep. 2016;12:281–7.
Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, Stange DE, et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459:262–5.
Van De Wetering M, Francies HE, Francis JM, Bounova G, Iorio F, Pronk A, et al. Prospective derivation of a living organoid biobank of colorectal cancer patients. Cell. 2015;161:933–45.
Xie BY, Wu AW. Organoid culture of isolated cells from patient-derived tissues with colorectal cancer. Chin Med J. 2016;129:2469–75.
Drost J, van Jaarsveld RH, Ponsioen B, Zimberlin C, van Boxtel R, Buijs A, et al. Sequential cancer mutations in cultured human intestinal stem cells. Nature. 2015;521:43–7.
Barrett CW, Reddy VK, Short SP, Motley AK, Lintel MK, Bradley AM, et al. Selenoprotein P influences colitis-induced tumorigenesis by mediating stemness and oxidative damage. J Clin Invest. 2015;125:2646–60.
Kiraly O, Gong G, Olipitz W, Muthupalani S, Engelward BP. Inflammation-induced cell proliferation potentiates DNA damage-induced mutations in vivo. PLoS Genet. 2015;11:e1004901.
• Nozaki K, Mochizuki W, Matsumoto Y, Matsumoto T, Fukuda M, Mizutani T, et al. Co-culture with intestinal epithelial organoids allows efficient expansion and motility analysis of intraepithelial lymphocytes. J Gastroenterol. 2016;51:206–13. Describes co-culture of intestinal epithelial organoids with intraepithelial lymphocytes (IELs).
Pastuła A, Middelhoff M, Brandtner A, Tobiasch M, Höhl B, Nuber AH, et al. Three-dimensional Gastrointestinal organoid culture in combination with nerves or fibroblasts: a method to characterize the Gastrointestinal stem cell niche. Stem Cells Int. 2016;2016:3710836.
Rogoz A, Reis BS, Karssemeijer RA, Mucida D. A 3-D enteroid-based model to study T-cell and epithelial cell interaction. J Immunol Methods. 2015;421:89–95.
Shaffiey SA, Jia H, Keane T, Costello C, Wasserman D, Quidgley M, et al. Intestinal stem cell growth and differentiation on a tubular scaffold with evaluation in small and large animals. Regen Med. 2015;11:rme.15.70.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Apple G. Long, Emma T. Lundsmith, and Kathryn E. Hamilton declare they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Basic Science Foundations in Colorectal Cancer
Rights and permissions
About this article
Cite this article
Long, A.G., Lundsmith, E.T. & Hamilton, K.E. Inflammation and Colorectal Cancer. Curr Colorectal Cancer Rep 13, 341–351 (2017). https://doi.org/10.1007/s11888-017-0373-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11888-017-0373-6