Abstract
Purpose of Review
Pericardial effusion is a challenging pericardial syndrome and a cause of serious concern for physicians and patients due to its potential progression to life-threatening cardiac tamponade. In this review, we summarize the contemporary evidence of the etiology; diagnostic work-up, with particular emphasis on the contribution of multimodality imaging; therapeutic options; and short- and long-term outcomes of these patients.
Recent Findings
In recent years, an important piece of information has contributed to put together several missing parts of the puzzle of pericardial effusion. The most recent 2015 guidelines of the European Society of Cardiology for the diagnosis and management of pericardial diseases are a valuable aid for a tailored approach to this condition. Actually, current guidelines suggest a 4-step treatment algorithm depending on the presence or absence of hemodynamic impairment; the elevation of inflammatory markers; the presence of a known or first-diagnosed underlying condition, possibly related to pericardial effusion; and finally the duration and size of the effusion. In contrast to earlier perceptions, based on the most recent evidence, it seems that in the subgroup of asymptomatic patients with large (> 2-cm end-diastolic diameter), chronic (> 3 months) C-reactive protein negative, idiopathic (without an apparent cause) pericardial effusion, a conservative approach is the most reasonable option.
Summary
At present there is an increasing interest in the pericardial syndromes in general and pericardial effusions in specific, which has consistently expanded our knowledge in this “hazy landscape.” Apart from general recommendations applied to all cases, an individualized, etiologically driven treatment is of paramount importance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pericardial effusion is defined as the abnormal accumulation of fluid within the pericardial cavity which normally does not exceed 50 ml [1••, 2••]. Along with acute pericarditis (first episode or recurrences), cardiac tamponade and constrictive pericarditis (transient, permanent, and effusive-constrictive) constitute the most common pericardial syndromes encountered in clinical practice [3,4,5]. From a pathophysiological perspective, possible conditions responsible for the development of pericardial effusion encompass pericardial fluid overproduction, which is actually the case of ongoing pericardial inflammation, trauma, decreased reabsorption mainly due to neoplastic invasion of lymphatic vessels, and finally an imbalance between hydrostatic and colloid osmotic pressures (e.g., heart failure, liver cirrhosis, and nephrotic syndrome) [2••, 6].
In recent years, there is an uprising interest concerning pericardial syndromes in general and pericardial effusion in specific. The most recent 2015 Europeans Society of Cardiology (ESC) guidelines for the diagnosis and management of pericardial diseases summarize the contemporary knowledge on pericardial syndromes [1••]. Νonetheless, a consistent new piece of information after the release of the guidelines has been presented in the international literature affecting our decision-making and clinical practice. In this context, the purpose of this review is to present the current evidence on pericardial effusion management, as well as discuss the perspective and unmet needs.
Epidemiology, Classification, and Etiology
Available data on the epidemiology of pericardial effusions are scant. In the Western world, the estimated incidence and prevalence are 3% and 5.7–9%, respectively, whereas pertinent data from the developing countries where the leading underlying etiology is tuberculosis are lacking [7, 8].
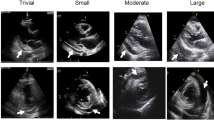
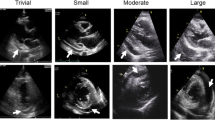
Pericardial effusions may be classified according to their size, duration, composition, distribution, etiology, and hemodynamic impact with each parameter being important to the overall patients’ management [2••]. The size of pericardial effusion is estimated semi-quantitatively with echocardiography by measuring the largest end-diastolic echo-free space (Fig. 1). It should be emphasized that separation of pericardial layers observed exclusively during systole in the setting of a routine echocardiographic examination (namely trivial effusion) represents a normal and clinically insignificant amount of fluid (< 50 ml). Effusions with an end-diastolic diameter less than 1 cm are classified as mild (~ 100 ml), those with diameter > 1 cm and < 2 cm as moderate (100–500 ml), and finally those exceeding 2 cm as large [1,2,••–3]. Effusions lasting < 1 week are classified as acute, subacute if lasting between 1 week and 3 months, and chronic when present for more than 3 months [1••]. Traditionally the criteria adopted for the characterization of pericardial effusions in transudates and exudates were Light’s criteria applied for pleural effusions [9]. However, recent data depicted that the above criteria cannot and should not be used for that purpose. Indeed, a relevant investigation showed that since normal pericardial fluid is rich in nucleated cells, albumin, protein, and lactate dehydrogenase (LDH), it is misclassified as an exudate in most instances [10]. Thus, biochemical analysis of pericardial fluid should not rely on the classical Light’ criteria [3, 10]. It is self-explanatory that specific criteria applying for pericardial fluid are eagerly awaited.
Transthoracic echocardiography in an asymptomatic subject with chronic, large (2.4-cm maximum end-diastolic diameter—double-head, green arrows), C-reactive protein–negative pericardial effusion, without evidence of hemodynamic impairment. A and B Parasternal long-axis and short-axis view, respectively, depicting large pericardial effusion in the posterior pericardial space. C and D Four chamber and subxiphoid view, respectively, showing large circumferential pericardial effusion. E Trans-mitral Doppler interrogation revealing normal respiratory variation pattern. F M-mode image showing normal size and inspiratory collapse (> 50%) of the inferior vena cava. PE = pericardial effusion, E = peak early filling, A = peak late filling, IVC = inferior vena cava
Other parameters used for the classification of pericardial effusion include distribution and hemodynamic impact. Due to gravity forces, pericardial effusions typically first appear posteriorly in the parasternal long-axis echocardiographic view and anteriorly in the area adjacent to the right atrium in the 4-chamber view [2••]. As fluid continues to accumulate, the effusion becomes circumferential. Loculated effusions may be occasionally observed following cardiac surgery or pericardial inflammation of any etiology with septa formation. Effusions may be asymptomatic or may have an impact (more or less severe) on cardiac hemodynamics, depending on the rate of accumulation.
The pericardial effusion etiology includes infectious and non-infectious causes [1••, 2••]. In the Western world, the most common cause of pericardial effusion is acute idiopathic-presumed viral pericarditis and in developing countries tuberculosis (> 70%) [4]. It is emphasized that pericardial effusion is observed in 60–80% of acute pericarditis cases [4, 11, 12]. Echovirus, coxsackievirus, parvovirus B19, and human herpesvirus 6 are the most commonly encountered viruses with variations depending on the local epidemiology [2••]. Notably in the era of the coronavirus (COVID-19) pandemic, pericardial involvement is reported in a number of studies. In the latter cases, pericardial involvement is observed in ~ 19% of cases, mostly in the setting of severe disease with multi-organ system failure [13]. However, several cases with isolated pericardial effusion have been described with occasional presence of positive COVID-19 PCR in the pericardial fluid. In a systematic review of cardiac magnetic resonance (CMR) findings in COVID-19 patients, pericardial effusion (any size) was detected in 24% of patients and in particular in those most severely affected [14]. On the other hand, non-infectious cases include autoimmune and autoinflammatory diseases, cancer, metabolic disorders, mediastinal radiation, post-traumatic pericarditis, aortic diseases, certain medications, and hemodynamic disorders altering the balance between hydrostatic and colloid osmotic pressures [2••]. Nevertheless, in up to 50% of cases in the high-income countries, the etiology of the effusion remains undetected, with these cases being finally labeled as idiopathic [1••].
Clinical Presentation and Diagnostic Work-Up
Pericardial effusion clinical manifestations range from symptom-free cases to critical, depending mainly on the rate of accumulation and also on the etiology [2••]. For instance, in rapidly accumulated fluid, the time for pericardium to stretch is insufficient and heart chambers (mostly the right ones due to the thinner-more compressible wall) collapse during diastole because of the high intrapericardial pressures. Thus, diastolic filling is impaired, the preload of the left ventricle is reduced, and finally cardiac output decreases [3]. Typical symptoms include dyspnea on exertion, fullness, orthopnea, and, in the presence of pericarditis, pericarditic chest pain [1••, 2••]. In the case of tamponade, the classical symptoms referred as Beck’s triad (i.e., hypotension, raised jugular venous pressure, and muffled heart sounds) along with tachycardia and pulsus paradoxus are present [1••, 15].
The initial approach in a patient presenting with pericardial effusion includes personal and family medical history, clinical examination, electrocardiography, routine blood tests including C-reactive protein (CRP) and troponin, as well as chest x-ray and echocardiography [2••, 3]. Chest radiography is not specific since it may depict enlargement of cardiac silhouette in at least moderate effusions (> 300 ml) but has the advantage to unravel a concomitant pleural effusion and pulmonary or mediastinal pathology [16, 17]. Electrocardiographic findings have a relatively low sensitivity and specificity and consist of low QRS voltage and electrical alternans in larger size effusions [3]. In effusions appearing in the setting of acute pericarditis, sinus tachycardia, diffuse concave ST-segment elevation, and PR-segment depression with absence of q waves may be among others detected [12].
Echocardiography is the mainstay for the diagnosis, quantification, and detection of the impact of pericardial effusion on heart hemodynamics [1••, 18••]. It is readily available, safe, and inexpensive and can be performed at bedside. In the case of near or overt cardiac tamponade, echocardiography reveals a large effusion with diastolic collapse of the right heart chambers, inferior vena cava plethora (> 20 mm) with reduced inspiratory collapse (< 50%), increased respiratory variation in E wave velocity during respiration across the mitral valve (> 25–30%) and tricuspid valve (> 50–60%), as well as expiratory diastolic flow reversal in the Doppler recording of hepatic venous flow velocity [1••, 2••]. It should however be emphasized that large pericardial effusion is not an equivalent of cardiac tamponade and chronic large effusions may be well tolerated (Fig. 1).
In recent years, multimodality imaging including computed tomography (CT) and cMR depicts a pivotal role in the evaluation of pericardial diseases, with particular emphasis to the difficult cases [18••, 19]. Chest CT is able to detect extracardiac lesions, pericardial thickness, presence of calcifications, and focal pericardial effusions and gives valuable information on the composition of the fluid based on the attenuation values (Hounsfield units—HU). Attenuation values < 10 HU denote transudative fluids while those > 10 HU exudative [3, 20]. Values between 20 and 60 HU suggest a purulent, malignant, or myxedematous etiology, whereas those > 60 HU suggest hemorrhagic fluid. Finally, values of − 60 to − 80 HU are reported in cases of chylopericardium [1••, 3, 20]. CMR on the other hand has the unique advantage of tissue characterization and evaluation of inflammation. Notably cMR is able to determine the degree of inflammation. A prominent late gadolinium enhancement (LGE) with an increased signal in T2-weighted sequences is associated with intense acute inflammation. In contrast, an increased LGE with a normal T2 signal is suggestive of a subacute/chronic low-grade inflammation, characterized by edema resolution [21•, 22]. Positron emission tomography (PET) and PET/CT have emerged as a useful clinical tool for diagnostic and prognostic purposes in pericardial diseases. In the specific context of pericardial effusion, 18fluorodeoxyglucose (18FDG) PET/CT unveils intense metabolic activity in cases of malignant spread to the pericardium and in inflammatory pericardial effusion [23]. Moreover, PET/CT may contribute to the differential diagnosis between tuberculous and “idiopathic” pericarditis with the former yielding higher FDG uptakes [1••, 23]. Finally, PET/CT may guide treatment length by assessing response to treatment. According to the ESC guidelines, chest CT, cMR, and PET-PET/CT are considered as second-line tests for the diagnostic approach of pericardial effusions and should be performed in an individualized manner [1••]. However, since in more than half of patients with moderate and large effusions a specific cause is identified, a lower threshold for multimodality imaging should be probably set [3]. The selection of imaging technique for the individual patient should take into account the clinical scenario and the laboratory findings. In brief, CT is the procedure of choice for the diagnosis of extracardiac disease, for the evaluation of pericardial thickness and calcification, as well as for preoperative planning. Thus, in the context of pericardial effusions, patients with effusive-constrictive pericarditis and those with suspicion of extracardiac disease on clinical grounds are ideal candidates for CT [18••]. On the other hand, cMR, based on its ability of superior tissue characterization and evaluation of pericardial inflammation, is the ideal method for the detection of ongoing pericardial inflammation and treatment guidance [1••, 18••]. In addition, cMR depicts excellent diagnostic accuracy for the detection of constrictive physiology on free breathing cine sequences. Thus, patients with possible malignant pericarditis (e.g., patients with a known malignancy presenting with pericardial effusion) and those with suspicion of transient or permanent constrictive pericarditis are good candidates for this imaging technique. Moreover, both CT and cMR should be performed for the diagnosis of pericardial cysts and diverticula. Finally, PET/CT should be reserved for challenging cases when the above-mentioned diagnostic methods do not provide a definite diagnosis (such as neoplastic disease and tuberculosis) and may be also helpful in establishing prognosis [18••, 23].
The role of cardiac catheterization for the diagnosis of pericardial syndromes has been downgraded in the most recent ESC guidelines. This is obviously due to the wide availability and use of advanced imaging techniques in the everyday clinical practice. Thus, cardiac catheterization that was traditionally the gold standard for the diagnosis of constrictive pericarditis, at present, is indicated when non-invasive diagnostic methods do not provide a definite diagnosis of constriction (class I recommendation, level of evidence C) [1••]. End-diastolic pressure equalization (< 5 mmHg) of the left and right ventricles (square root or dip and plateau sign) and ventricular interdependence depicted by a systolic area index > 1.1 are the key findings of constrictive physiology [1••]. In cardiac tamponade, equalization of mean right atrial, right ventricular, mean pulmonary artery diastolic pressure, and pulmonary capillary wedge pressures is observed. Cardiac catheterization is also useful for the challenging differentiation between constrictive pericarditis and restrictive cardiomyopathy and for the detection of eventual coronary artery disease in patients with an indication for cardiac surgery.
Management of Patients with Pericardial Effusion
The 2015 ESC Guidelines on Pericardial Diseases recommend a very useful simplified diagnostic algorithm for patients with pericardial effusions targeting therapy at the etiology and hemodynamic impact [1••]. The proposed algorithm consists of four fundamental steps. The first step refers to patients presenting with cardiac tamponade, suspicion of neoplastic or bacterial etiology (including tuberculosis) on clinical grounds. These patients should be treated with pericardial drainage (pericardiocentesis or pericardial window depending on effusion characteristics and local expertise) for therapeutic and diagnostic purposes [1••, 2••]. In the specific context of cardiac tamponade, a scoring system has been developed to assess the timing of pericardiocentesis. In particular, by assessing etiology, clinical presentation, and imaging findings, a cumulative score is obtained. Values ≥ 6 dictate urgent pericardiocentesis, while with a score < 6, pericardiocentesis may be postponed for 12–48 h allowing transfer of the patient to a referral specialized center (Table 1) [15]. It is emphasized that for safety reasons pericardiocentesis should be always echo or fluoroscopy guided [24]. In echocardiography-monitored pericardiocentesis, the most appropriate entry site is the one closer to the largest amount of the effusion, with subxiphoid, apical, and parasternal sites being the most widely adopted entry sites [2••]. CT-guided pericardiocentesis constitutes an effective and safe alternative option. It may be performed by experienced centers especially in specific clinical scenarios (e.g., loculated effusions, symptomatic pericardial cysts). CT guidance offers the advantage of a better evaluation of the needle direction and positioning in relation to adjacent anatomic structures [25]. Importantly, a rapid evacuation of more than 1 l of fluid during pericardiocentesis should be avoided in order to prevent pericardial decompression syndrome, which is a potentially fatal complication manifesting with either pulmonary edema or cardiogenic shock [15, 26].
In 16% of patients undergoing pericardiocentesis for cardiac tamponade, constrictive physiology may persist after pericardiocentesis [27]. In this condition, cardiac constriction by a thickened visceral pericardial layer coexists with tense pericardial effusion. Thus, pericardial drainage fails to reduce right atrial pressure by at least 50% or to a value below 10 mmHg [1••]. In this condition, prognosis is overall good if cases with underlying malignancy are excluded. Notably, in contrast with classical permanent constrictive pericarditis, a small number of cases (~ 12%) who do not respond to anti-inflammatory therapy will eventually require pericardiectomy [27].
In cases that a purulent effusion is drained, apart from systemic antibiotic therapy intrapericardial fibrinolysis, or pericardial window with rinsing, debridement and drainage of infected fluid should be considered in an individualized basis [3, 28]. In patients with effusion of malignant etiology, a multidisciplinary approach is required with cooperation of specialists. Intrapericardial instillation of cytostatic/sclerosing agents has been proven to be effective for the management of malignant effusions (IIa level of evidence B according to the ESC guidelines) [1••, 29]. In the setting of malignant pericardial effusion, based on a recent investigation, administration of colchicine after extended drainage has been associated with a lower rate of the composite of all-cause death and repeated pericardial drainage [30•]. It should be emphasized that bloody effusions in patients presenting with cardiac tamponade underlie a newly discovered malignancy in ~ 2% of cases [31]. This rate rises at ~ 26% in the context of patients with a known malignancy. On the other hand, hemorrhagic effusion is observed in ~ 62% of cases of acute viral pericarditis [32]. Thus, hemorrhagic effusions should not be perceived a priori as a marker of malignancy. Last but not least in patients undergoing pericardiocentesis, the draining catheter should be left in place until less than 30 ml of fluid/24 h is drained [1••, 15]. Prolonged drainage has been recently shown to induce local inflammation which may account for pericardial space obliteration and less fluid re-accumulation although further data are required to support this hypothesis [33].
In the absence of any of the aforementioned clinical scenarios, the second step of the relevant ESC algorithm recommends inflammatory markers’ measurement (namely, CRP). In case of CRP elevation, this subgroup of patients should be treated with the protocol of acute pericarditis [1••]. In accordance with the contemporary ESC recommendations, aspirin or non-steroidal anti-inflammatory drugs along with colchicine and gastroprotection should be administered as first-line treatment. Glucocorticoids are prescribed as a second choice in cases with contraindications or not tolerability to first-line medications or whenever there is a specific indication for this treatment (e.g., autoimmune disease) [1••, 34, 35].
On the condition that there is no indication for emergent pericardiocentesis and CRP values are in the normal range, the next (third) step recommends a triage for eventual medical conditions potentially accounting for pericardial effusion. If such a condition is unveiled during work-up, then management should be targeted to the underlying etiology and a multidisciplinary approach is required. It is worth noting that in patients with a moderate or large pericardial effusion, a secondary condition is present (either known or unveiled during work-up) in ~ 50–60% of cases [1••, 3, 4, 36].
Regarding hemodynamic causes of pericardial effusion, non-inflammatory transudative effusions may occur in hypoalbuminemia, heart failure, and pulmonary arterial hypertension, with the common denominator in the last two conditions being an increase in systemic venous pressure due to right heart failure [1••]. Pericardial effusion is observed in ~ 8.5% of patients with chronic heart failure and in up to 30% of patients with pulmonary arterial hypertension [1••, 37]. It should be emphasized that both heart failure and pulmonary hypertension almost never progress to cardiac tamponade but in both instances, they portend a poor prognosis [1••, 15]. Notably some of the typical features of cardiac tamponade may be absent in pulmonary hypertension (such as absence of right heart chambers diastolic collapse due to the elevated right-sided pressures, but also pulsus paradoxus and arterial hypotension) [1••, 38]. In general, small effusions in pulmonary hypertension may be managed medically, whereas treatment of large pericardial effusions is controversial since occasional deaths have been reported after pericardiocentesis [38].
Finally, the fourth and last step of the algorithm refers probably to the most problematic group of patients, namely, those with idiopathic, CRP-negative pericardial effusions without (or with minimal) hemodynamic consequences. In this subgroup, stable small to moderate effusions do not require a specific intervention and should be simply followed up every ~ 6 months [1••]. In contrast, in cases of large effusions especially if lasting more than 3 months, pericardiocentesis should be considered for therapeutic and diagnostic purposes. The latter recommendation was essentially based on a small-sized study including 28 patients, published ~ 20 years earlier which reported a progression to cardiac tamponade in approximately 1/3 of cases [39]. However, these concerns were not confirmed in a lager recent investigation which included 100 similar patients [40••]. In this investigation, after a mean follow-up of 50 months, it was found that the rate of progression of large chronic CRP-negative pericardial effusion to overt cardiac tamponade is 2.2%/year. Remarkably the effusion during follow-up was reduced in size in the majority of patients and regressed spontaneously in ~ 40% of cases. Pericardiocentesis was required during the study period in about one-third of patients. Event-free survival (cardiac tamponade and any pericardial intervention) did not differ between patients with or without symptoms at baseline. Moreover, event-free survival (complications and recurrences) was actually worse in the subgroup of patients subjected to interventions (pericardiocentesis, pericardial window, and pericardiectomy) compared with those treated conservatively (log rank test p = 0.0038). As a result, the authors discouraged a routine pericardial effusion drainage in this population of patients and recommended a tailored clinical and echocardiographic follow-up instead.
Another pertinent study addressed the outcome of 52 asymptomatic patients (while the above-mentioned study enrolled both symptomatic and asymptomatic patients) with chronic, idiopathic, CRP-negative, hemodynamically insignificant pericardial effusions undergoing pericardiocentesis or pericardial window [41]. Pericardial drainage was performed either due to concerns for a gradually increasing size of the effusion or for diagnostic purposes. After a median follow-up of 24 months, fluid re-accumulation was detected in ~ 77% of patients undergoing pericardiocentesis with large re-accumulation occurring in 41% of cases. The relevant rates of patients undergoing pericardial window were 15.4 and 7.7%, respectively. Patients with re-accumulation after pericardial drainage had larger effusion volume drained at baseline, higher maximum end-diastolic effusion size, and longer disease duration. Since a comprehensive baseline diagnostic work-up was performed in all cases, pericardial drainage was not helpful in unraveling new diagnoses and administering specific treatments. A non-negligible rate of complications (both intraprocedural and during the observation period) was recorded in both types of interventional procedures (12.8% and 15.4% of patients who underwent pericardiocentesis and pericardial window, respectively). In the same study, in a subgroup of additional 22 patients who opted for conservative treatment, pericardial effusion remained overall stable during follow-up in 17 cases (77.3%), regressed in 3 cases (13.6%), whereas 2 patients (9.1%)) experienced near cardiac tamponade and underwent pericardial drainage. Cardiac tamponade occurred in 4 out of 52 patients in the intervention group (i.e., 7.7% of patients, with 2 cases during pericardiocentesis and the remainder during follow-up).
In light of the recent evidence, a more conservative approach with a tailored follow-up seems reasonable in asymptomatic patients with long-standing, large asymptomatic effusions. In this line, the fourth step recommendations of the guidelines for this subcategory of patients may be updated as shown in Fig. 2.
Prognosis and Follow-Up
The outcome of patients presenting with pericardial effusion varies largely depending on the etiology and effusion size [1••, 2••]. Idiopathic effusions and pericardial effusions appearing in the course of acute pericarditis have an overall good prognosis, especially concerning those with small and moderate size [1••, 2••]. Nevertheless, a study showed that the presence of even small asymptomatic pericardial effusion was independently associated with increased mortality (hazard ratio (HR) 1.17) [8]. Moderate to large effusions in approximately half of cases appear in the context of a secondary condition which may affect prognosis [3]. Finally, in a pertinent meta-analysis, it was concluded that pericardial effusion should be considered as a marker of severity of the underlying disease and the risk of death was higher in patients with effusions, independently of the primary disorder (HR 1.59) [42].
The follow-up of patients with pericardial effusion should take into account several parameters including effusion size and duration, elevation of inflammatory markers, and presence of symptoms. Patients with first-detected effusions should be carefully monitored for effusion stability every 1–2 weeks after the initial diagnosis and then after a month. In case that effusion size remains unaffected, follow-up may be scheduled every 6 months [2••].
Chronic mild effusions not causing symptoms do not require specific follow-up based on experts’ opinion [2••, 3]. Moderate-sized effusions should be assessed every 6 months and large effusions every 3–6 months [1••, 2••]. Patients with effusions adjacent to the easily compressible thin right heart chambers require closer follow-up and a lower threshold for drainage, should symptoms appear [3, 41]. In each follow-up visit, clinical examination and a focused echocardiographic assessment, ideally in a specialized referral center, should be performed. It is again stressed that patients should be educated to seek medical advice if new symptoms appear.
Conclusion
Pericardial effusion is a common and sometimes troublesome pericardial syndrome. Prognosis largely depends on the underlying etiology which emphasizes the paramount importance of a detailed diagnostic work-up. Treatment should be individualized, taking into account the clinical features, presence of inflammation, comorbidities, and effusion size. Although management should definitely comply with the current guideline recommendations, new piece of evidence should be taken into account in the clinical decision-making.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
•• Adler Y, Charron P, Imazio M, Badano L, Barón-Esquivias G, Bogaert J, et al. ESC Guidelines for the diagnosis and management of pericardial diseases. Eur Heart J. 2015;36(42):2921–64 This is the full text of the most recent guidelines for the diagnosis and management of patients with pericardial diseases.
•• Imazio M, Adler Y. Management of pericardial effusion. Eur Heart J. 2013;34(16):1186–97 Comprehensive review depicting the contemporary evidence of the management of pericardial effusion.
Lazaros G, Vlachopoulos C, Lazarou E, Tousoulis D, Tsioufis C. Contemporary management of pericardial effusion. Panminerva Med. 2021. https://doi.org/10.23736/S0031-0808.20.04197-X.
Imazio M, Spodick DH, Brucato A, Trinchero R, Adler Y. Controversial issues in the management of pericardial diseases. Circulation. 2010;121(7):916–28.
Lazaros G, Imazio M, Brucato A, Tousoulis D. Untying the Gordian knot of pericardial diseases: a pragmatic approach. Hell J Cardiol. 2010;57(5):315–22.
Vogiatzidis K, Zarogiannis SG, Aidonidis I, Solenov EI, Molyvdas PA, Gourgoulianis KI, et al. Physiology of pericardial fluid production and drainage. Front Physiol. 2015;6:62.
Imazio M, Mayosi BM, Brucato A, Markel G, Trinchero R, Spodick DH, et al. Triage and management of pericardial effusion. J Cardiovasc Med (Hagerstown). 2010;11(12):928–35.
Mitiku TY, Heidenreich PA. A small pericardial effusion is a marker of increased mortality. Am Heart J. 2011;161(1):152–7.
Light RW, Macgregor MI, Luchsinger PC, Ball WC Jr. Pleural effusions: the diagnostic separation of transudates and exudates. Ann Intern Med. 1972;77(4):507–13.
Buoro S, Tombetti E, Ceriotti F, Simon C, Cugola D, Seghezzi M, et al. What is the normal composition of pericardial fluid? Heart. 2020 Nov 11:heartjnl-2020-317966. https://doi.org/10.1136/heartjnl-2020-317966.
Lazaros G, Solomou E, Antonopoulos AS, Vlachopoulos C, Vasileiou P, Karavidas A, et al. The landscape of acute pericarditis in Greece: experience from a tertiary referral center. Hell J Cardiol. 2019;60(2):139–40.
Imazio M, Gaita F. Diagnosis and treatment of pericarditis. Heart. 2015;101(14):1159–68.
Basso C, Leone O, Rizzo S, De Gaspari M, van der Wal AC, Aubry MC, et al. Pathological features of COVID-19-associated myocardial injury: a multicentre cardiovascular pathology study. Eur Heart J. 2020;41(39):3827–35.
Ojha V, Verma M, Pandey NN, Mani A, Malhi AS, Kumar S, et al. Cardiac magnetic resonance imaging in coronavirus disease 2019 (COVID-19): a systematic review of cardiac magnetic resonance imaging findings in 199 Patients. J Thorac Imaging. 2020 Dec 9;36:73–83. https://doi.org/10.1097/RTI.0000000000000574.
Ristić AD, Imazio M, Adler Y, Anastasakis A, Badano LP, Brucato A, et al. Triage strategy for urgent management of cardiac tamponade: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2014;35(34):2279–84.
Eisenberg MJ, Dunn MM, Kanth N, Gamsu G, Schiller NB. Diagnostic value of chest radiography for pericardial effusion. J Am Coll Cardiol. 1993;22(2):588–93.
Lazaros G, Antonopoulos AS, Imazio M, Solomou E, Lazarou E, Vassilopoulos D, et al. Clinical significance of pleural effusions and association with outcome in patients hospitalized with a first episode of acute pericarditis. Intern Emerg Med. 2019;14(5):745–51.
•• Klein AL, Abbara S, Agler DA, Appleton CP, Asher CR, Hoit B, et al. American Society of Echocardiography clinical recommendations for multimodality cardiovascular imaging of patients with pericardial disease: endorsed by the Society for Cardiovascular Magnetic Resonance and Society of Cardiovascular Computed Tomography. J Am Soc Echocardiogr. 2013;26(9):965–1012.e15 Comprehensive guide on the role of multimodality imaging in the assessment of pericardial diseases.
Chetrit M, Xu B, Verma BR, Klein AL. Multimodality Imaging for the Assessment of Pericardial Diseases. Curr Cardiol Rep. 2019;21(5):41.
Verhaert D, Gabriel RS, Johnston D, Lytle BW, Desai MY, Klein AL. The role of multimodality imaging in the management of pericardial disease. Circ Cardiovasc Imaging. 2010;3(3):333–43.
• Chetrit M, Xu B, Kwon DH, Ramchand J, Rodriguez RE, Tan CD, et al. Imaging-guided therapies for pericardial diseases. JACC Cardiovasc Imaging. 2020;13(6):1422–37 This study highlights the role of multimodality imaging in the care of patients with pericardial disease.
Cremer PC, Kumar A, Kontzias A, Tan CD, Rodriguez ER, Imazio M, et al. Complicated pericarditis: understanding risk factors and pathophysiology to inform imaging and treatment. J Am Coll Cardiol. 2016;68(21):2311–28.
Kim MS, Kim EK, Choi JY, Oh JK, Chang SA. Clinical Utility of [18F]FDG-PET /CT in Pericardial Disease. Curr Cardiol Rep. 2019;21(9):107.
Lazaros G, Imazio M, Tousoulis D. Percutaneous pericardiocentesis: safety first! Cardiology. 2015;130(1):34–6.
Neves D, Silva G, Morais G, Ferreira N, Carvalho M, Gama Ribeiro V, et al. Computed tomography-guided pericardiocentesis - a single-center experience. Rev Port Cardiol. 2016;35(5):285–90.
Imazio M. Pericardial decompression syndrome: a rare but potentially fatal complication of pericardial drainage to be recognized and prevented. Eur Heart J Acute Cardiovasc Care. 2015;4(2):121–3.
Kim KH, Miranda WR, Sinak LJ, Syed FF, Melduni RM, Espinosa RE, et al. Effusive-constrictive pericarditis after pericardiocentesis: incidence, associated findings, and natural history. JACC Cardiovasc Imaging. 2018;11(4):534-41.
Wiyeh AB, Ochodo EA, Wiysonge CS, Kakia A, Awotedu AA, Ristic A, et al. A systematic review of the efficacy and safety of intrapericardial fibrinolysis in patients with pericardial effusion. Int J Cardiol. 2018;250:223–8.
Ala CK, Klein AL, Moslehi JJ. Cancer treatment-associated pericardial disease: epidemiology, clinical presentation, diagnosis, and management. Curr Cardiol Rep. 2019;21(12):156.
• Kim SR, Kim EK, Cho J, Chang SA, Park SJ, Lee SC, et al. Effect of anti-inflammatory drugs on clinical outcomes in patients with malignant pericardial effusion. J Am Coll Cardiol. 2020;76(13):1551–61 This study showed that patients receiving colchicine after successful pericardiocentesis showed significant improvement in clinical outcome.
Atar S, Chiu J, Forrester JS, Siegel RJ. Bloody pericardial effusion in patients with cardiac tamponade: is the cause cancerous, tuberculous, or iatrogenic in the 1990s? Chest. 1999;116(6):1564–9.
Meyers DG, Meyers RE, Prendergast TW. The usefulness of diagnostic tests on pericardial fluid. Chest. 1997;111(5):1213–21.
Lazaros G, Oikonomou V, Oikonomou E, Aznaouridis K, Vlachopoulos C, Vogiatzi G, et al. Recurrence of pericardial effusion after pericardiocentesis. Does catheter-induced acute pericardial inflammation play a role? Am J Med Sci. 2020 Oct 12:S0002-9629(20)30445-6. https://doi.org/10.1016/j.amjms.2020.10.012.
Lazaros G, Tousoulis D, Vassilopoulos D. Editorial commentary: recurrent pericarditis in the era of interleukin-1 inhibition. Trends Cardiovasc Med. 2020;14:S1050-1738(20)30064-5. https://doi.org/10.1016/j.tcm.2020.04.010.
Lazaros G, Antonopoulos AS, Vlachopoulos C, Oikonomou E, Karavidas A, Chrysochoou C, et al. Predictors of switching from nonsteroidal anti-inflammatory drugs to corticosteroids in patients with acute pericarditis and impact on clinical outcome. Hell J Cardiol. 2019;60(6):357–63.
Sagrista-Sauleda J, Merce J, Permanyer-Miralda G, Soler-Soler J. Clinical clues to the causes of large pericardial effusions. Am J Med. 2000;109(2):95–101.
Fröhlich GM, Keller P, Schmid F, Wolfrum M, Osranek M, Falk C, et al. Haemodynamically irrelevant pericardial effusion is associated with increased mortality in patients with chronic heart failure. Eur Heart J. 2013;34(19):1414–23.
Sahay S, Tonelli AR. Pericardial effusion in pulmonary arterial hypertension. Pulm Circ. 2013;3(3):467–77.
Sagristà-Sauleda J, Angel J, Permanyer-Miralda G, Soler-Soler J. Long-term follow-up of idiopathic chronic pericardial effusion. N Engl J Med. 1999;341(27):2054–9.
•• Imazio M, Lazaros G, Valenti A, De Carlini CC, Maggiolini S, Pivetta E, et al. Outcomes of idiopathic chronic large pericardial effusion. Heart. 2019;105(6):477–81 This is the largest study published at present assessing the outcome of patients with chronic large effusions in the absence of inflammation.
Lazaros G, Antonopoulos AS, Lazarou E, Vlachopoulos C, Foukarakis E, Androulakis A, et al. Long-term outcome of pericardial drainage in cases of chronic, large, hemodynamically insignificant, C-Reactive Protein Negative. Idiopathic Pericardial Effusions Am J Cardiol. 2020;126:89–93.
De Filippo O, Gatti P, Rettegno S, Iannaccone M, D'Ascenzo F, Lazaros G, et al. Is pericardial effusion a negative prognostic marker? Meta-analysis of outcomes of pericardial effusion. J Cardiovasc Med (Hagerstown). 2019;20(1):39–45.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Pericardial Disease
Rights and permissions
About this article
Cite this article
Lazaros, G., Vlachopoulos, C., Lazarou, E. et al. New Approaches to Management of Pericardial Effusions. Curr Cardiol Rep 23, 106 (2021). https://doi.org/10.1007/s11886-021-01539-7
Accepted:
Published:
DOI: https://doi.org/10.1007/s11886-021-01539-7