Abstract
Purpose of Review
The COVID-19 pandemic has forced many center-based cardiac rehabilitation (CBCR) programs to close or limit their usual offerings. In order for patients to continue to benefit from CR, programs need to rapidly adapt to the current environment. This review highlights ways CR has evolved, and reviews the history of CR and recent advancements in telemedicine including remote patient monitoring, and mobile health that can be applied to CR.
Recent Findings
Despite that initial studies indicate that home-based CR (HBCR) is safe and effective, HBCR has faced several challenges that have prevented it from becoming more widely implemented. Many previous concerns can now be addressed through the use of new innovations in home-based healthcare delivery.
Summary
Since its inception, CR has become increasingly recognized as an important tool to improve patient mortality and quality of life in a broad range of cardiac diseases. While there has been little need to modify the delivery of CR since the 1950s, COVID-19 now serves as the necessary impetus to make HBCR an equal alternative to CBCR.
Similar content being viewed by others
Introduction
Evolution of Cardiac Rehabilitation into the Standard of Care
At the start of the twentieth century, patients were not expected to recover after myocardial infarction (MI). With the presumption of long-term convalescence, common management was strict bedrest. As physicians in the 1950s began to realize the benefits of early mobilization, cardiac rehabilitation became the treatment of choice with the new expectation that patients would return to their prior level of activity [1]. This shift can be attributed to physicians such as Herman Hellerstein, who developed a revolutionary understanding of the influence of lifestyle factors on cardiovascular risk. To target those lifestyle factors and improve functioning, Hellerstein created the first multidisciplinary CR program and urged physicians to recognize their essential role in rehabilitation beyond diagnosis and treatment of disease, writing that “the practice of rehabilitation should not be below the dignity of the physician” [2].
While technology and pharmacotherapy for the treatment of cardiovascular disease (CVD) have evolved dramatically since the 1950s, and lifestyle modification is now recognized as the first-line treatment, CVD has remained the leading cause of death worldwide. In 2016, CVD was responsible for over 9.5 million deaths worldwide [3–4]. As a result, the importance of CR has become increasingly clear. Although Hellerstein’s approach was once considered radical, in 1994, the American Heart Association (AHA) emphasized that CR should be the standard of care and integrated into the treatment plan for all patients with CAD [5]. The AHA also defined the goals of CR: to improve functional capacity, reduce disability, and identify and modify coronary risk factors in an attempt to reduce subsequent morbidity and mortality due to cardiovascular illness. A year later, in 1995, the Agency for Healthcare Policy and Research released Cardiac Rehabilitation, which pioneered a framework of comprehensive healthcare guidelines regarding integration of medical management, exercise, and education for those living with CVD in the USA. These emphasized exercise training, physical activity, risk reduction, and pharmacotherapy [6].
Although from its inception the goal of CR was to improve a patient’s functional status and quality of life, there has since been an accumulation of outcome data demonstrating a mortality benefit [7,8,9]. As a result, the AHA/American College of Cardiology (ACC) has given CR a class 1A recommendation. In addition, data continues to accumulate showing the benefits of CR to patient function and quality of life for a broad range of indications beyond coronary artery disease (CAD). Accordingly, over time the number of indications for CR has expanded to include prior heart valve repair or replacement (including newer technologies such as transcatheter aortic valve replacement (TAVR) and MitraClip, prior heart transplant, heart-lung transplant, or left ventricular assist device (LVAD)), systolic congestive heart failure New York Heart Association class II to IV, and peripheral arterial disease [10]. There is a growing body of data showing that CR is beneficial for patients with heart failure with preserved ejection fraction (HFPEF) [11,12]. Though HFPEF is not currently a covered indication for CR, there is lobbying from professional societies based on recent data to expand coverage to include this important population of patients.
Center-Based Cardiac Rehabilitation
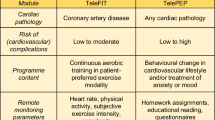
CR in the USA is delivered primarily via in-person, center-based programs (center-based cardiac rehabilitation, or CBCR) in two formats: traditional cardiac rehabilitation (TCR) and a newer model, intensive cardiac rehabilitation (ICR) (Fig. 1). Two widely accepted models of ICR are the Pritikin Program and the Ornish Reversal Program [10]. Both TCR and ICR are reimbursed by the Centers for Medicare and Medicaid Services (CMS) and other third-party payers. Home-based cardiac rehabilitation (HBCR), on the other hand, is still in its infancy in the USA.
TCR follows a 36-session model with 3 sessions per week for 12 weeks. These sessions consist of moderate-intensity aerobic exercises in addition to education on CVD and lifestyle modifications [6,13]. For further risk reduction, TCR includes education on tobacco and alcohol cessation. Individual cardiometabolic risk factors are evaluated comprehensively with assessment of lipid panels, HBA1c, blood pressure (BP), and weight [14].
ICR programs involve comprehensive lifestyle changes and incorporate modalities such as stress management, group therapy, and nutrition counseling. The Pritikin Program is formatted with 36 h of aerobic exercise and 36 h of educational sessions [15]. Participants are encouraged to consume a low-fat diet with an emphasis on vegetables, grains, and fruit, while saturated fats and cholesterol-rich foods such as egg yolks and red meat are discouraged. The exercise component includes aerobic exercises as well as strength and flexibility training, citing studies showing that the combination yields increased peak oxygen consumption, a measure of cardiorespiratory fitness [16]. The program also incorporates mental health practices and stress management classes.
The Ornish program core components include nutrition, fitness, stress management, and group support. For nutrition, consumption of healthy, monounsaturated fats and plant-based proteins and limitation of alcohol, sugars, and sodium is recommended [17]. The program does not allow for consumption of oil or animal products other than egg whites. The fitness component involves 60-min sessions of monitored, moderate-intensity aerobic exercise. Yoga and meditation are encouraged for stress management. Patients also engage in group support and psychosocial counseling to create a strong foundation of mental health. The Lifestyle Heart Trial demonstrated that the Ornish program resulted in a 37.2% reduction in low-density lipoprotein (LDL) cholesterol, a 91% reduction in anginal episodes, and a 2.2% regression in average percent diameter coronary artery stenosis. Additionally, it significantly increased myocardial perfusion as compared to the control group [17]. Another randomized trial (n = 93) demonstrated that patients in the Ornish program had significantly greater improvements in weight, dietary habits, and body mass index (BMI) compared to TCR (p < .001) [18].
The Challenge of COVID-19 for Center-Based Cardiac Rehabilitation
Among the many challenges that COVID-19 poses worldwide is the challenge to the delivery of CR via in-person, CBCR. At the same time, COVID-19 has only highlighted the important role CR plays in empowering patients to make healthy lifestyle choices that will reduce their risk of ASCVD and COVID-19-related morbidity and mortality [19].
Despite its reliable benefits to mortality and quality of life and despite improvement in referral rates, only 34% of referred patients enrolled in CR prior to COVID-19. Only 16.3% of Medicare patients and 10.3% of veterans participated in CR after hospitalization for MI, percutaneous coronary intervention, or coronary artery bypass graft surgery [20]. In particular, women and minorities are disproportionately represented in CR. Women are 36% less likely to be enrolled and less likely to complete the program compared to men [21]. Black, Hispanic, and Asian patients were 20%, 36%, and 50% less likely, respectively, to receive CR referral compared to white patients [22]. Factors that contribute to this overall low enrollment rate include a poor centralized method for referral, inadequate communication between treatment teams, and perceived inconvenience for the patient [10]. During the pandemic, additional requirements for testing, inflexible scheduling for essential workers, and financial burden may exacerbate the already existing barriers.
COVID-19 may enhance existing gaps in access to healthcare in general. Even without the stress imposed by the pandemic, socioeconomic status has been linked to underutilization of CR [21]. Now, an additional 7.3 million workers and their families may become uninsured during the pandemic because of unemployment [23]. As a result, many low-income patients will lose access to CR and therefore remain at a higher risk of ASCVD with a greater number of cardiometabolic risk factors. The disproportionate number of racial and ethnic minorities participating in CR may be one reason why COVID-19 disproportionately affects these groups [24].
Both TCR and ICR are more commonly delivered via a center-based approach (CBCR). These programs require patients to be physically present at a facility located in a hospital or outpatient center. Most CBCR programs have shut down in-person activities to limit the potential for COVID-19 transmission. Those that remain open must substantially decrease the number of patients in the space to enforce social distancing guidelines, making it difficult to accommodate all referred patients and continue the standard CR activities.
COVID-19 has not only made access to CR challenging for patients, but centers themselves face a multitude of challenges. Many centers find that CR during COVID-19 is not financially sustainable. Less patients attending CR limit revenue, limit the number of staff that can be maintained, and in turn further limit the number of patients that can be safely accommodated. Centers located in rural and community hospitals receive fewer referrals to CR, have lower participation at baseline, and therefore may be disproportionately impacted by COVID-19 [25].
Closely supervised exercise is an important component of CBCR and is currently required by CMS [10]. Healthcare providers are present for direct observation and coaching of patients during exercise, and telemetry and BP monitoring are employed to ensure that patients are exerting themselves safely [13,26]. Although severe cardiac events during CBCR are rare, the safety of remote patient exercise monitoring has not been thoroughly studied [27,28]. This makes it difficult for existing CBCR programs to swiftly transition to a home-based model.
COVID-19 also interferes with the ability of CR patients to connect and benefit from the support of their peers. Typically, ICR programs include meal sessions, yoga for stress management, and support sessions—all in a group environment to foster camaraderie. Such group support is important for patient morale and has been shown to greatly enhance patient adherence [10]. Often, CR cohorts create lasting bonds and continue to support each other once the program has finished. Given these aspects of CR would be absent under most county COVID-19 guidelines, new approaches are needed to attain a similar sense of group connection virtually.
After 30 years with little change in CR delivery, this is an opportunity for innovation so that patients can continue to benefit from CR in a safe manner. While HBCR programs do exist, they are rare, not as widely studied, and therefore lack clear guidelines for implementation. The use of new technologies to create a safe and effective HBCR program will not just allow CR to continue during the pandemic, but may also lead to higher utilization rates in the future.
Home-Based Cardiac Rehabilitation
HBCR programs have long been available in the UK, Canada, Australia, and even rarely in the USA (Fig. 1). These programs were created in an attempt to overcome some of the barriers that prevent patients from participating in CBCR programs, including geographic, logistical, and other access-related barriers.
A recent review article examined 23 randomized controlled trials (RCTs) of HBCR and CBCR and found that these programs tend to implement the same core components [29]. These include patient assessment, exercise training, dietary counseling, risk factor management (smoking, lipids, BP, weight, diabetes mellitus), and psychological intervention. Comparison of studies in HBCR and CBCR indicates a similar improvement in quality of life and no statistically significant difference in all-cause mortality up to 12 months after the intervention [30,32,33,34,35,36,37,37]. However, the majority of these studies included low-risk patients after uncomplicated myocardial infarction. It is unclear how HBCR compares to CBCR in other patient groups, particularly high-risk patients such as LVAD and transplant patients.
Most HBCR programs involve home walking programs supported by telephone calls or home visits by an exercise physiologist or nurse [29]. Some programs employed heart rate monitors and others remote electrocardiographic telemetry monitoring. The HF-ACTION trial provided treadmills or stationary bicycles in addition to a heart rate monitor to the HBCR group, which resulted in modest adherence [38]. Through these methods, HBCR has been shown to result in a similar improvement in peak oxygen uptake compared to CBCR [29]. In one study (n = 30), peak oxygen uptake increased 4.6 (±2.7) and 3.9 (±3.6) mL·kg−1 min−1 (both p < 0.005, non-significant between-group difference) in CBCR and HBCR respectively [32].
A recent RCT in Europe (n = 179) demonstrated that 6 months of HBCR in adults aged 65 or older with CAD or valvular disease was both successful in improving cardiorespiratory fitness and safe [39••]. The participants had all refused referral to CBCR. They received a heart rate monitor and a smart phone with a special application designed for the study. Patients always wore heart rate monitors while exercising and were coached via telephone using motivational interviewing techniques based on their individual recorded exercise data. Peak oxygen uptake increased after 6 months (1.6 [95% CI, 0.9 to 2.4] mL/kg−1/min−1; relative increase of 8.5%) and 12 months (1.2 [95% CI, 0.4 to 2.0] mL/kg−1/min−1; relative increase of 6.3%) in patients participating in HBCR, whereas no changes were observed for the control group. Similarly, patients completing HBCR self-reported a greater increase in physical activity compared to the control group at 6 months (+1.2 [95% CI, 0.2 to 2.1] mL/kg−1/min−1) and 12 months (+0.9 [95% CI, 0.05 to 1.8] mL/kg−1/min−1). Importantly, the incidence of adverse events did not differ between the HBCR and control groups (12 of 89 [13%] vs. 10 of 90 [11%]; p = 0.66). This RCT demonstrates that an exercise-based CR program can be safely implemented in the home setting and can enact lasting changes in the behavior and functioning of a group of patients who had previously refused CBCR.
Beyond exercise training, HBCR programs offer dietary counseling through a variety of methods, including telephone, weekly educational and counseling meetings, home visits, dietary counseling sessions and practice cooking sessions, educational materials, or a web portal or smartphone [29]. Similar to CBCR, psychological support and stress management are also components of HBCR. They also employ a variety of strategies to improve lifestyle habits, including smoking cessation, and adherence to prescribed medications.
One of the main challenges that has prevented HBCR from becoming more widely implemented is lack of reimbursement. Following an executive order to improve rural health and telehealth access, CMS has now expanded the list of telehealth services that Medicare Fee-For-Service will pay for during COVID-19 to include cardiac and pulmonary rehabilitation services [40]. The executive order went further to call for the expansion of healthcare delivery flexibility beyond the public health emergency. Given that telehealth visits have continued to be frequent despite that in-person visits have now resumed, remote visits are likely to be a permanent feature of healthcare delivery.
Other challenges faced by HBCR include less intensive exercise training, less social support, less patient accountability, lack of published standards, less face-to-face monitoring and communication, and safety concerns for high-risk patients [29]. Using existing HBCR models as a basis, many of these concerns can potentially be addressed by new innovations in home-based healthcare delivery.
New Innovations in Home-Based Healthcare Delivery
The Rise of Telemedicine
COVID-19 has already rapidly transformed telemedicine from a rarely used alternative into common practice. An in-depth patient interview can now easily be performed via HIPAA-compliant video sessions integrated into the electronic medical record. During the first quarter of 2020, telemedicine visits increased by 50% compared with the same time period in 2019 [41]. Large medical systems have trained their staff to be proficient in virtual-based encounters, and many insurance companies now cover virtual visits.
In addition to empowering patients to examine themselves and employing caregivers to assist in examination, aspects of the physical exam can also be performed at home using new technologies [42]. The FDA-approved Eko stethoscope incorporates a remote feature which enables accurate remote auscultation of patients and simultaneous electrocardiogram (EKG) capture [43]. The patient places the capturing end of the stethoscope on his or her chest, while the clinician is able to hear high-fidelity audio in real-time and visualize audio waveforms and a single-lead EKG. There is also the option to utilize artificial intelligence algorithms to assist in predicting whether a patient is at risk for heart disease with greater accuracy. Several algorithms are now FDA approved to detect valvulopathies and arrhythmias. One algorithm has been shown to detect low ejection fraction <35% and mitral regurgitation based on the EKG obtained with the Eko [44,45].
Even laboratory (lab) testing may eventually be possible at home. Over the last 5 years, companies such as LabCorp, EverlyWell, LetsGetChecked, MyLabBox, and 23andme have developed home lab testing kits [46]. These tests are not just convenient, but they improve patient access, remove burden on the healthcare system, and decrease the risk of exposure to infection. While they currently incur out-of-pocket costs, it is possible that these services could be employed in the future for home lab monitoring of HBCR patients.
Remote Patient Monitoring
There are several FDA-approved cardiac monitors which have been used in clinical care for cardiac monitoring up to 14 days. Recently, auscultatory devices have been integrated into garments for long-term monitoring [47]. Alternatively, implanted, long-term cardiac monitors have been developed which can detect S3 heart sounds and predict heart failure exacerbations with greater accuracy than auscultation with a stethoscope [48]. With further study, these technologies have the potential to make supervision of home exercise easier and safer, similar to how the CardioMEMs pulmonary artery pressure monitoring device has been shown to significantly reduce hospitalizations in patients with heart failure with both reduced and preserved ejection fraction [49].
Popular commercial wearable devices that monitor heart rate can be used to assess cardiorespiratory fitness and allow for remote monitoring of patient safety. These devices can be found in the form of a watch, bracelet, or ring. The Apple watch can transmit a single-lead EKG and blood oxygen levels (SpO2). It is able to notify the patient about heart bradycardia, tachycardia, or irregular rhythms and is also able to sense when a patient may have fallen and notify emergency services. It is FDA approved to detect atrial fibrillation with a sensitivity of 98.0% and specificity of 90.2% [50]. The watch has also been used to measure QTc adequately in 85% of patients when worn on the left wrist [51]. It may even be possible to diagnose acute coronary syndromes based on ECGs recorded on smart watches [52]. While they possess great potential, these technologies need to undergo rigorous clinical trials in order to demonstrate that they improve outcomes.
Continuous glucose monitors (CGMs) have also seen dramatic improvement, now with the availability of wearable monitors that do not require confirmatory finger sticks. These CGMs have been shown to improve glycemic control in patients with type 1 diabetes [53,55,55]. Now also approved in type 2 diabetes (T2DM), these provide instant feedback on patients’ dietary choices. CGMs have also been shown to reduce hypoglycemia in older adults [56]. The data from these monitors can be tracked remotely by physicians and by patients on their smart phone or watch. Given 20-44% of patients referred to CR have T2DM, this technology could be instrumental in making HBCR safer for higher risk patient groups [57,58].
Technologies such as the CGM need to be curated and integrated into CR programs in a meaningful way. To avoid overwhelming patients, they should be incorporated into the electronic medical record with a use-friendly dashboard. In addition, programs will need to work with payors in order to get coverage for these devices so that they can be provided to CR patients for the duration of the program.
Mobile Health
Mobile health (mHealth) is defined as the use of wireless technologies to improve health outcomes [59]. Patient-generated data has come a long way from having a patient record their BP readings with a pen and paper. Now, there are more than 250,000 mobile health applications available and more than 300 million wearable devices in use [60]. In a feasibility study examining patient use of virtual-based visits and mHealth, including a BP monitor, step counter, weight scale, and a single-lead EKG, 63% of patients transmitted data in more than 80% of the 52-week study [61•]. Additionally, of those who participated, patient satisfaction scores were similar to those who had usual care. This study suggests that patients participating in CR would likely be able to successfully generate wireless health data for the duration of the program and have similar satisfaction compared to CBCR.
Now that patients are generating this incredible amount of data, the question becomes how we use the information to improve health outcomes. mHealth has been successfully utilized to mitigate several ASCVD risk factors. A mobile application has been used to monitor dietary patterns and ultimately modulate those patterns to achieve reduced body weight, increased energy, and improved sleep [62]. In a systematic review and meta-analysis of eleven randomized controlled trials investigating mHealth for BP management, mHealth resulted in significant reductions in systolic BP and diastolic BP compared to control [63]. Several studies have indicated that mHealth can improve management of diabetes [64]. While several studies have examined various lifestyle interventions via mHealth with the goal of managing hyperlipidemia, there is not yet a consistent benefit [65].
mHealth has also been used to increase patient exercise by improving motivation and self-efficacy and providing objective targets [64]. The HEART trial (n = 171), which utilized a personalized, automated package of text messages and a secure website with video messages aimed at increasing exercise behavior, resulted in increased physical activity [66]. Wearable devices can more eloquently assess cardiorespiratory fitness with the assistance of validated algorithms to interpret the data. Using data from the HUNT Fitness Study, an algorithm was developed which used continuous heart rate data to create a personal activity score that can be trended over time [67]. Several studies have shown this score to correlate with cardiac risk. A score ≥100 had a reduced risk of mortality (17% [7-27%] in men and 23% [4-38%] in women, p < 0.01) and was associated with a reduced risk of death due to cardiovascular disease (p < 0.01). A subsequent study showed that obtaining a score ≥100 attenuated the positive association of sedentary behavior with clustering of risk factors for CVD [68]. In addition, a score ≥100 was associated with higher maximum oxygen consumption in both men (4.1 mL/kg min; 95% CI, 3.5 to 4.6) and women (2.9 mL/kg min; 95% CI, 2.4 to 3.3), compared to the reference group of <100 [69]. Scores such as this could serve as objective, noninvasive mechanisms for physicians to monitor patients’ cardiorespiratory fitness in the outpatient setting.
One study (n = 80) evaluated the use of mHealth in CR specifically and demonstrated improved outcomes in CBCR patients who used a smartphone application (app) compared to those who did not [70••]. Patients using the app had greater weight loss compared to the control group (−5.1 ± 6.5 kg vs. −0.8 ± 3.8 kg, respectively, p = 0.02). In addition, there was a trend towards reduced CV-related rehospitalizations plus ED visits compared to the control group at 180 days. This study demonstrates the potential of mHealth to improve outcomes in CR patients. The challenge will be to robustly integrate telemedicine, remote patient monitoring, and mHealth into existing CR models in order to achieve a program that is effective, safe, user-friendly, and reimbursable by insurance (Fig. 1).
Hybrid Model of Cardiac Rehabilitation
The hybrid CR model involves 1-2 initial sessions in the center and subsequent sessions completed remotely with home-based monitoring. The initial in-person sessions provide the opportunity to ensure safety and psychosocial well-being, increase participation in group sessions, and individualize patients’ home-based exercise programs. Studies have shown that this model is safe and yields comparable results to CBCR. In one systematic review and meta-analysis (n = 1195), CR delivered through the hybrid model was found to result in a similar improvement in functional capacity (SMD = −0.04, 95% CI −0.18 to 0.09, p = 0.51). In addition, no significant difference was detected between the two models in terms of changes in exercise duration (SMD = −0.14, 95% CI −0.51 to 0.24, p = 0.47), systolic BP (SMD = −0.01, 95% CI −0.14 to 0.12, p = 0.91), diastolic BP (SMD = −0.03, 95% CI −0.16 to 0.11, p = 0.7), or health-related quality of life (SMD = −0.08, 95% CI −0.23 to 0.07, p = 0.27) [71]. Another systematic review and meta-analysis (n = 1791) demonstrated that hybrid CR resulted in a greater improvement in peak oxygen uptake compared to usual care (9.72 mL/kg per minute; 95% CI, 5.12–14.33) [72•]. CR may evolve towards a hybrid model that strikes an ideal balance between CBCR and HBCR (Fig. 1).
Conclusions
COVID-19 serves as both a challenge to CBCR and a long-awaited impetus to increase CR utilization. With successful existing HBCR programs serving as a basis, advancements in telemedicine, remote patient monitoring, and patient-generated data via mobile health have the potential to create a safe, effective, and standardized home-based alternative to CBCR. The explosion in new technologies such as CGMs and smartphone applications could be tailored to make home-based programs safe and effective. This process will require testing these new technologies within CR and creating evidence-based guidelines for HBCR programs. At the same time, there is value in the center-based approach—in hands-on teaching, in coaching, and in human connection—and there may be evolution towards a hybrid model. It is time to chart a new course to an accessible and adaptable CR program with the goal of improving access to CR both now and in the future.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Pashkow FJ. Issues in contemporary cardiac rehabilitation: a historical perspective. J Am Coll Cardiol. 1993;21:822–34.
Hellerstein HK, Ford AB. Rehabilitation of the cardiac patient. J Am Med Assoc. 1957;164:225–31.
FastStats. 2020. https://www.cdc.gov/nchs/fastats/leading-causes-of-death.htm.
The top 10 causes of death. https://www.who.int/en/news-room/fact-sheets/detail/the-top-10-causes-of-death.
Balady GJ, et al. Cardiac rehabilitation programs. Circulation. 1994;90:1602–7.
Horgan J, Bethell H, et al. Working party report on cardiac rehabilitation. Br Heart J. 1992;67:412–8.
Anderson L, Oldridge N, et al. Exercise-based cardiac rehabilitation for coronary heart disease: Cochrane systematic review and meta-analysis. J Am Coll Cardiol. 2016;67:1–12.
Rauch B, Davos CH. The prognostic effect of cardiac rehabilitation in the era of acute revascularisation and statin therapy: a systematic review and meta-analysis of randomized and non-randomized studies--The Cardiac Rehabilitation Outcome Study (CROS). Eur J Prev Cardiol. 2016;23:1914–39.
de Vries H, Kemps HMC, van Engen-Verheul MM, Kraaijenhagen RA, Peek N. Cardiac rehabilitation and survival in a large representative community cohort of Dutch patients. Eur Heart J. 2015;36:1519–28.
Freeman AM, Taub PR, Lo HC, Ornish D. Intensive cardiac rehabilitation: an underutilized resource. Curr Cardiol Rep. 2019;21:19.
Edelmann F, et al. Exercise training improves exercise capacity and diastolic function in patients with heart failure with preserved ejection fraction: results of the Ex-DHF (Exercise training in Diastolic Heart Failure) pilot study. J Am Coll Cardiol. 2011;58:1780–91.
Klempfner R, et al. Participation in an exercise-based cardiac rehabilitation program and functional improvement of heart failure patients with preserved versus reduced left ventricular systolic function. Isr Med Assoc J. 2018;20:358–62.
Carlson JJ, Johnson JA, Franklin BA, VanderLaan RL. Program participation, exercise adherence, cardiovascular outcomes, and program cost of traditional versus modified cardiac rehabilitation. Am J Cardiol. 2000;86:17–23.
Smith Sidney C, et al. AHA/ACCF secondary prevention and risk reduction therapy for patients with coronary and other atherosclerotic vascular disease: 2011 update. Circulation. 2011;124:2458–73.
Businessweek B, et al. Pritikin Health Resort | Voted Best Weight Loss Resort. https://www.pritikin.com/.
Smart N, Marwick TH. Exercise training for patients with heart failure: a systematic review of factors that improve mortality and morbidity. Am J Med. 2004;116:693–706.
Ornish D, et al. Intensive lifestyle changes for reversal of coronary heart disease. JAMA. 1998;280:2001–7.
Dominique W, et al. Abstract P325: effectiveness of the first outpatient Pritikin Intensive Cardiac Rehabilitation (ICR) program. Circulation. 2019;139:AP325.
Khera A, et al. Continuity of care and outpatient management for patients with and at high risk for cardiovascular disease during the COVID-19 pandemic: a scientific statement from the American Society for Preventive Cardiology. Am J Prev Cardiol. 2020;1:100009.
Beatty AL, et al. Geographic variation in cardiac rehabilitation participation in Medicare and veterans affairs populations: opportunity for improvement. Circulation. 2018;137:1899–908.
Schultz WM, et al. Socioeconomic status and cardiovascular outcomes: challenges and interventions. Circulation. 2018;137:2166–78.
Shanshan L, et al. Sex and racial disparities in cardiac rehabilitation referral at hospital discharge and gaps in long-term mortality. J Am Heart Assoc. 2018;7(8). https://doi.org/10.1161/JAHA.117.008088
Woolhandler S, Himmelstein DU. Intersecting U.S. epidemics: COVID-19 and lack of health insurance. Ann Intern Med. 2020;173:63–4.
CDC. COVID-19 cases, deaths, and trends in the US. 2020. https://covid.cdc.gov/covid-data-tracker/.
Aragam KG, et al. Gaps in referral to cardiac rehabilitation of patients undergoing percutaneous coronary intervention in the United States. J Am Coll Cardiol. 2015;65:2079–88.
Franklin BA, Reed PS, Gordon S, Timmis GC. Instantaneous electrocardiography. A simple screening technique for cardiac exercise programs. Chest. 1989;96:174–7.
Haskell WL. The efficacy and safety of exercise programs in cardiac rehabilitation. Med Sci Sports Exerc. 1994;26:815–23.
Pavy B, et al. Safety of exercise training for cardiac patients: results of the French registry of complications during cardiac rehabilitation. Arch Intern Med. 2006;166:2329–34.
Thomas RJ, et al. Home-based cardiac rehabilitation: a scientific statement from the American Association of Cardiovascular and Pulmonary Rehabilitation, the American Heart Association, and the American College of Cardiology. J Am Coll Cardiol. 2019;74:133–53.
Miller NH, Haskell WL, Berra K, DeBusk RF. Home versus group exercise training for increasing functional capacity after myocardial infarction. Circulation. 1984;70:645–9.
Bell JM. A comparison of a multi-disciplinary home based cardiac rehabilitation programme with comprehensive conventional rehabilitation in post-myocardial infarction patients. 1998. https://spiral.imperial.ac.uk/bitstream/10044/1/8585/1/Jennifer_M_Bell-1999-PhD-Thesis.pdf
Moholdt T, Bekken Vold M, Grimsmo J, Slørdahl SA, Wisløff U. Home-based aerobic interval training improves peak oxygen uptake equal to residential cardiac rehabilitation: a randomized, controlled trial. PLoS One. 2012;7:e41199.
Daskapan A, Arikan H, Caglar N, Tunali N, Ataman S. Comparison of supervised exercise training and home-based exercise training in chronic heart failure. Saudi Med J. 2005;26:842–7.
Oerkild B, et al. Home-based cardiac rehabilitation is as effective as centre-based cardiac rehabilitation among elderly with coronary heart disease: results from a randomised clinical trial. Age Ageing. 2011;40:78–85.
Piotrowicz E, et al. A new model of home-based telemonitored cardiac rehabilitation in patients with heart failure: effectiveness, quality of life, and adherence. Eur J Heart Fail. 2010;12:164–71.
Dalal HM, et al. Home-based versus hospital-based rehabilitation after myocardial infarction: a randomized trial with preference arms--Cornwall Heart Attack Rehabilitation Management Study (CHARMS). Int J Cardiol. 2007;119:202–11.
Jolly K, et al. The Birmingham Rehabilitation Uptake Maximisation study (BRUM): a randomised controlled trial comparing home-based with centre-based cardiac rehabilitation. Heart. 2009;95:36–42.
O’Connor CM, et al. Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA. 2009;301:1439–50.
•• Snoek JA, et al. Effectiveness of home-based mobile guided cardiac rehabilitation as alternative strategy for nonparticipation in clinic-based cardiac rehabilitation among elderly patients in Europe: a randomized clinical trial. JAMA Cardiol. 2020. https://doi.org/10.1001/jamacardio.2020.5218This randomized controlled trial in 179 patients demonstrates that home-based cardiac rehabilitation can be safely and effectively implemented in a group of patients who had previously refused referral to center-based cardiac rehabilitation.
Trump Administration Drives Telehealth Services in Medicaid and Medicare. https://www.cms.gov/newsroom/press-releases/trump-administration-drives-telehealth-services-medicaid-and-medicare.
Koonin LM, et al. Trends in the use of Telehealth during the emergence of the COVID-19 pandemic-United States, January-March 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1595–9.
Hollander Judd E, Sites Frank D. The transition from reimagining to recreating health care is now. Catalyst non-issue content 1(2). https://doi.org/10.1056/CAT.20.0093
Behere S, Baffa JM, Penfil S, Slamon N. Real-world evaluation of the Eko electronic teleauscultation system. Pediatr Cardiol. 2019;40:154–60.
Attia Zachi I, et al. Abstract 13447: prospective analysis of utility of signals from an Ecg-enabled stethoscope to automatically detect a low ejection fraction using neural network techniques trained from the standard 12-lead Ecg. Circulation. 2019;140:A13447.
White Brent E, et al. Abstract 13831: handheld wireless digital phonocardiography for machine learning-based detection of mitral regurgitation. Circulation. 2019;140:A13831.
Croteau J. Can at-home lab testing answer your health needs? Forbes Magazine. 2019. https://www.forbes.com/sites/jeannecroteau/2019/09/30/can-at-homelab-testing-answer-your-health-needs/. Accessed Sept 30 2019.
Yilmaz G, et al. A wearable stethoscope for long-term ambulatory respiratory health monitoring. Sensors. 2020;20(18). https://doi.org/10.3390/s20185124
Cao M, et al. Ambulatory monitoring of heart sounds via an implanted device is superior to auscultation for prediction of heart failure events. J Card Fail. 2020;26:151–9.
Abraham WT, et al. Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: a randomised controlled trial. Lancet. 2011;377:658–66.
Tison GH, et al. Passive detection of atrial fibrillation using a commercially available smartwatch. JAMA Cardiol. 2018;3:409–16.
Strik M, et al. Validating QT-interval measurement using the Apple watch ECG to enable remote monitoring during the COVID-19 pandemic. Circulation. 2020;142:416–8.
Spaccarotella CAM, et al. Multichannel electrocardiograms obtained by a smartwatch for the diagnosis of ST-segment changes. JAMA Cardiol. 2020. https://doi.org/10.1001/jamacardio.2020.3994.
Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group. Effectiveness of continuous glucose monitoring in a clinical care environment: evidence from the Juvenile Diabetes Research Foundation continuous glucose monitoring (JDRF-CGM) trial. Diabetes Care. 2010;33:17–22.
Beck RW, et al. Effect of continuous glucose monitoring on glycemic control in adults with type 1 diabetes using insulin injections: the DIAMOND randomized clinical trial. JAMA. 2017;317:371–8.
Battelino T, et al. The use and efficacy of continuous glucose monitoring in type 1 diabetes treated with insulin pump therapy: a randomised controlled trial. Diabetologia. 2012;55:3155–62.
Pratley RE, et al. Effect of continuous glucose monitoring on hypoglycemia in older adults with type 1 diabetes: a randomized clinical trial. JAMA. 2020;323:2397–406.
Ettefagh L, et al. The prevalence of impaired glucose metabolism in patients referred to cardiac rehabilitation. J Cardiopulm Rehabil Prev. 2013;33:42–6.
Mourot L, et al. Cardiovascular rehabilitation in patients with diabetes. J Cardiopulm Rehabil Prev. 2010;30:157–64.
mHealth: new horizons for health through mobile technologies. World Health Organization. https://www.who.int/goe/publications/goe_mhealth_web.pdf.
Paper, White. n.d. Conceptualizing a data infrastructure for the capture, use, and sharing of patient-generated health data in care delivery and research through 2024. https://www.healthit.gov/sites/default/files/onc_pghd_final_white_paper.pdf. Accessed Feb 25 2021.
• Treskes RW, et al. Effect of smartphone-enabled health monitoring devices vs regular follow-up on blood pressure control among patients after myocardial infarction: a randomized clinical trial. JAMA Netw Open. 2020;3:e202165. This randomized controlled trial demonstrates that patients can successfully utilize mobile health (mHealth) to generate data.
Gill S, Panda S. A smartphone app reveals erratic diurnal eating patterns in humans that can be modulated for health benefits. Cell Metab. 2015;22:789–98.
Lu X, Yang H, Xia X, Lu X, Lin J, Liu F, et al. Interactive mobile health intervention and blood pressure management in adults. Hypertension. (2019;74:697–704.
Rehman H, Kamal AK, Sayani S, Morris PB, Merchant AT, Virani SS. Using mobile health (mHealth) technology in the management of diabetes mellitus, physical inactivity, and smoking. Curr Atheroscler Rep. 2017;19:16.
Managing hyperlipidemia on the go: using mobile technology to lower cholesterol levels-American College of Cardiology. n.d. https://www.acc.org/latest-in-cardiology/articles/2017/08/03/08/27/managing-hyperlipidemia-on-the-go. Accessed Nov 15 2020.
Maddison R, Pfaeffli L, Whittaker R, Stewart R, Kerr A, Jiang Y, et al. A mobile phone intervention increases physical activity in people with cardiovascular disease: results from the HEART randomized controlled trial. Eur J Prev Cardiol. 2015;22:701–9.
Nes BM, Gutvik CR, Lavie CJ, Nauman J, Wisløff U. Personalized activity intelligence (PAI) for prevention of cardiovascular disease and promotion of physical activity. Am J Med. 2017;130:328–36.
Zisko N, Skjerve KN, Tari AR, Sandbakk SB, Wisløff U, Nes BM, et al. Personal activity intelligence (PAI), sedentary behavior and cardiovascular risk factor clustering-the HUNT Study. Prog Cardiovasc Dis. (2017;60:89–95.
Nauman J, Nes BM, Zisko N, Revdal A, Myers J, Kaminsky LA, et al. Personal activity intelligence (PAI): a new standard in activity tracking for obtaining a healthy cardiorespiratory fitness level and low cardiovascular risk. Prog Cardiovasc Dis. 2019;62:179–85.
•• Widmer RJ, et al. Digital health intervention during cardiac rehabilitation: a randomized controlled trial. Am Heart J. 2017;188:65–72 This randomized controlled trial demonstrates that mobile health can improve outcomes in cardiac rehabilitation.
Wu C, Li Y, Chen J. Hybrid versus traditional cardiac rehabilitation models: a systematic review and meta-analysis. Kardiol Pol. 2018;76:1717–24.
• Imran Hafiz M, et al. Home-based cardiac rehabilitation alone and hybrid with center-based cardiac rehabilitation in heart failure: a systematic review and meta-analysis. J Am Heart Assoc. 2019;8:e012779 This systematic review and meta-analysis demonstrates that the hybrid model of cardiac rehabilitation may result in a greater improvement in cardiorespiratory fitness compared to center-based cardiac rehabilitation (usual care).
Funding
Dr. Taub reports grants from NIH (R01 DK118278-01 and R01 HL136407), the American Heart Association (SDG #15SDG2233005), and the Department of Homeland Security/FEMA (EMW-2016-FP-00788).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Dr. Taub reports being a consultant for Amgen, Epirum Bio, Boehringer Ingelheim, Novo Nordisk, and Sanofi; and she is also a shareholder of Epirum Bio, outside the submitted work. The other authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Lipid Abnormalities and Cardiovascular Prevention
Rights and permissions
About this article
Cite this article
Epstein, E., Patel, N., Maysent, K. et al. Cardiac Rehab in the COVID Era and Beyond: mHealth and Other Novel Opportunities. Curr Cardiol Rep 23, 42 (2021). https://doi.org/10.1007/s11886-021-01482-7
Accepted:
Published:
DOI: https://doi.org/10.1007/s11886-021-01482-7