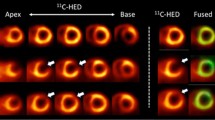
Abstract
Purpose of Review
The present article reviews the pathophysiology of cardiac sympathetic denervation, the principles of positron emission tomography (PET) imaging of the sympathetic innervation of the heart and its potential clinical role, based on current and expected future evidence.
Recent Findings
Imaging of cardiac sympathetic denervation can be performed with radiolabeled noradrenaline analogues, e.g., 11C-hydroxyephedrine. A greater burden of sympathetic denervation carries prognostic significance, e.g., in patients with ischemic cardiomyopathy and a left ventricular ejection fraction ≤ 35%, who are more likely to experience sudden cardiac death. Abnormalities of sympathetic cardiac innervation have been demonstrated in hypertrophic, dilated, and arrhythmic right ventricular cardiomyopathies, and may be helpful in better phenotyping patients who will benefit from device therapy, e.g., cardiac resynchronization and implantable cardioverter-defibrillator implantation. The results of future trials, e.g., the Prediction of Arrhythmic Events with Positron Emission Tomography (PAREPET) II study, are awaited to inform on the role of PET cardiac sympathetic imaging in the selection of device therapy.
Summary
PET cardiac sympathetic innervation imaging allows visualization and quantification of autonomic denervation secondary to various cardiac diseases, and has significant potential to influence clinical decision-making, e.g., the titration of pharmacotherapy and more directed selection of candidates for device implantation.




Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Haider N, Baliga RR, Chandrashekhar Y, Narula J. Adrenergic excess, hNET1 down-regulation, and compromised mIBG uptake in heart failure poverty in the presence of plenty. JACC Cardiovasc Imaging. 2010;3:71–5. https://doi.org/10.1016/j.jcmg.2009.11.002.
Schroeder C, Jordan J. Norepinephrine uptake mechanisms in cardiovascular disease deserve our attention. J Cardiovasc Pharmacol. 2011;58:406–8. https://doi.org/10.1097/FJC.0B013E31822EAE22.
Lautamaki R, Sasano T, Higuchi T, Nekolla SG, Lardo AC, Holt DP et al. Multiparametric molecular imaging provides mechanistic insights into sympathetic innervation impairment in the viable infarct border zone. J Nucl Med. 2015;56:457–63. :https://doi.org/10.2967/jnumed.114.149971.
Zhang DY, Anderson AS. The sympathetic nervous system and heart failure. Cardiol Clin. 2014;32:33–45, vii.:https://doi.org/10.1016/j.ccl.2013.09.010.
Liang CS. Cardiac sympathetic nerve terminal function in congestive heart failure. Acta Pharmacol Sin. 2007;28:921–7.:https://doi.org/10.1111/j.1745-7254.2007.00585.x.
•• Zelt JGE, Mielniczuk LM, Orlandi C, Robinson S, Hadizad T, Walter O, et al. PET imaging of sympathetic innervation with [(18)F]Flurobenguan vs [(11)C]mHED in a patient with ischemic cardiomyopathy. J Nucl Cardiol. 2019;26:2151–3. https://doi.org/10.1007/s12350-018-01527-5In this case report, the first comparative images for the novel radiotracer fluorobenguane and hydroxyephedrine in ischemic cardiomyopathy are published.
Fallavollita JA, Banas MD, Suzuki G, deKemp RA, Sajjad M, Canty JM, Jr. 11C-meta-hydroxyephedrine defects persist despite functional improvement in hibernating myocardium. J Nucl Cardiol. 2010;17:85–96.:https://doi.org/10.1007/s12350-009-9164-z.
Bengel FM, Permanetter B, Ungerer M, Nekolla SG, Schwaiger M. Relationship between altered sympathetic innervation, oxidative metabolism and contractile function in the cardiomyopathic human heart; a non-invasive study using positron emission tomography. Eur Heart J. 2001;22:1594–600.:https://doi.org/10.1053/euhj.2000.2556.
Martignani C, Diemberger I, Nanni C, Biffi M, Ziacchi M, Boschi S et al. Cardiac resynchronization therapy and cardiac sympathetic function. Eur J Clin Invest. 2015;45:792–9.:https://doi.org/10.1111/eci.12471.
Werner RA, Rischpler C, Onthank D, Lapa C, Robinson S, Samnick S et al. Retention kinetics of the 18F-labeled sympathetic nerve PET tracer LMI1195: comparison with 11C-hydroxyephedrine and 123I-MIBG. J Nucl Med. 2015;56:1429–33. :https://doi.org/10.2967/jnumed.115.158493.
Malizia AL, Melichar JK, Rhodes CG, Haida A, Reynolds AH, Jones T et al. Desipramine binding to noradrenaline reuptake sites in cardiac sympathetic neurons in man in vivo. Eur J Pharmacol. 2000;391:263–7.:https://doi.org/10.1016/s0014-2999(00)00103-5.
• Aikawa T, Naya M, Obara M, Manabe O, Tomiyama Y, Magota K et al. Impaired myocardial sympathetic innervation is associated with diastolic dysfunction in heart failure with preserved ejection fraction: (11)C-Hydroxyephedrine PET study. J Nucl Med. 2017;58:784-90. https://doi.org/10.2967/jnumed.116.178558. Findings from this study suggest that altered myocardial sympathetic innervation plays a role in the pathophysiology of heart failure with preserved ejection fraction.
Bengel FM. Imaging targets of the sympathetic nervous system of the heart: translational considerations. J Nucl Med. 2011;52:1167–70.:https://doi.org/10.2967/jnumed.110.084228.
Van der Bijl P, Delgado V, Bax JJ. Imaging for sudden cardiac death risk stratification: current perspective and future directions. Prog Cardiovasc Dis. 2019;62:205–11.:https://doi.org/10.1016/j.pcad.2019.04.005.
Fernandez SF, Ovchinnikov V, Canty JM, Jr., Fallavollita JA. Hibernating myocardium results in partial sympathetic denervation and nerve sprouting. Am J Physiol Heart Circ Physiol. 2013;304:H318–27.:https://doi.org/10.1152/ajpheart.00810.2011.
Fallavollita JA, Heavey BM, Luisi AJ, Jr., Michalek SM, Baldwa S, Mashtare TL, Jr. et al. Regional myocardial sympathetic denervation predicts the risk of sudden cardiac arrest in ischemic cardiomyopathy. J Am Coll Cardiol. 2014;63:141–9. :https://doi.org/10.1016/j.jacc.2013.07.096.
Van der Bijl P, Delgado V, Bax JJ. Sudden cardiac death: the role of imaging. Int J Cardiol. 2017;237:15–8.:https://doi.org/10.1016/j.ijcard.2017.03.010.
Narayanan K, Reinier K, Uy-Evanado A, Teodorescu C, Chugh H, Marijon E et al. Frequency and determinants of implantable cardioverter defibrillator deployment among primary prevention candidates with subsequent sudden cardiac arrest in the community. Circulation. 2013;128:1733–8.:https://doi.org/10.1161/CIRCULATIONAHA.113.002539.
Merlet P, Delforge J, Syrota A, Angevin E, Maziere B, Crouzel C et al. Positron emission tomography with 11C CGP-12177 to assess beta-adrenergic receptor concentration in idiopathic dilated cardiomyopathy. Circulation. 1993;87:1169–78. :https://doi.org/10.1161/01.cir.87.4.1169.
Li ST, Tack CJ, Fananapazir L, Goldstein DS. Myocardial perfusion and sympathetic innervation in patients with hypertrophic cardiomyopathy. J Am Coll Cardiol. 2000;35:1867–73.:https://doi.org/10.1016/s0735-1097(00)00626-4.
Schafers M, Dutka D, Rhodes CG, Lammertsma AA, Hermansen F, Schober O et al. Myocardial presynaptic and postsynaptic autonomic dysfunction in hypertrophic cardiomyopathy. Circ Res. 1998;82:57–62.:https://doi.org/10.1161/01.res.82.1.57.
Lefroy DC, de Silva R, Choudhury L, Uren NG, Crake T, Rhodes CG et al. Diffuse reduction of myocardial beta-adrenoceptors in hypertrophic cardiomyopathy: a study with positron emission tomography. J Am Coll Cardiol. 1993;22:1653–60. :https://doi.org/10.1016/0735-1097(93)90591-n.
Choudhury L, Guzzetti S, Lefroy DC, Nihoyannopoulos P, McKenna WJ, Oakley CM et al. Myocardial beta adrenoceptors and left ventricular function in hypertrophic cardiomyopathy. Heart. 1996;75:50–4.:https://doi.org/10.1136/hrt.75.1.50.
Wichter T, Schafers M, Rhodes CG, Borggrefe M, Lerch H, Lammertsma AA et al. Abnormalities of cardiac sympathetic innervation in arrhythmogenic right ventricular cardiomyopathy: quantitative assessment of presynaptic norepinephrine reuptake and postsynaptic beta-adrenergic receptor density with positron emission tomography. Circulation. 2000;101:1552–8.:https://doi.org/10.1161/01.cir.101.13.1552.
Kies P, Wichter T, Schafers M, Paul M, Schafers KP, Eckardt L et al. Abnormal myocardial presynaptic norepinephrine recycling in patients with Brugada syndrome. Circulation. 2004;110:3017–22.:https://doi.org/10.1161/01.CIR.0000146920.35020.44.
Vinik AI, Ziegler D. Diabetic cardiovascular autonomic neuropathy. Circulation. 2007;115:387–97.:https://doi.org/10.1161/CIRCULATIONAHA.106.634949.
Stevens MJ, Raffel DM, Allman KC, Schwaiger M, Wieland DM. Regression and progression of cardiac sympathetic dysinnervation complicating diabetes: an assessment by C-11 hydroxyephedrine and positron emission tomography. Metabolism. 1999;48:92–101.:https://doi.org/10.1016/s0026-0495(99)90016-1.
Hall AB, Ziadi MC, Leech JA, Chen SY, Burwash IG, Renaud J et al. Effects of short-term continuous positive airway pressure on myocardial sympathetic nerve function and energetics in patients with heart failure and obstructive sleep apnea: a randomized study. Circulation. 2014;130:892–901.:https://doi.org/10.1161/CIRCULATIONAHA.113.005893.
Deguchi K, Sasaki I, Tsukaguchi M, Kamoda M, Touge T, Takeuchi H et al. Abnormalities of rate-corrected QT intervals in Parkinson’s disease-a comparison with multiple system atrophy and progressive supranuclear palsy. J Neurol Sci. 2002;199:31–7. :https://doi.org/10.1016/s0022-510x(02)00079-5.
Wong KK, Raffel DM, Bohnen NI, Altinok G, Gilman S, Frey KA. 2-year natural decline of cardiac sympathetic innervation in idiopathic Parkinson disease studied with 11C-hydroxyephedrine PET. J Nucl Med. 2017;58:326–31. :https://doi.org/10.2967/jnumed.116.176891.
The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): a randomised trial. Lancet. 1999;353:9–13.
Ardell JL, Andresen MC, Armour JA, Billman GE, Chen PS, Foreman RD et al. Translational neurocardiology: preclinical models and cardioneural integrative aspects. J Physiol. 2016;594:3877–909. https://doi.org/10.1113/JP271869.
Ajijola OA, Wisco JJ, Lambert HW, Mahajan A, Stark E, Fishbein MC et al. Extracardiac neural remodeling in humans with cardiomyopathy. Circ Arrhythm Electrophysiol. 2012;5:1010–116. https://doi.org/10.1161/CIRCEP.112.972836.
Grimaldi R, de Luca A, Kornet L, Castagno D, Gaita F. Can spinal cord stimulation reduce ventricular arrhythmias? Heart Rhythm. 2012;9:1884–7. https://doi.org/10.1016/j.hrthm.2012.08.007
Bourke T, Vaseghi M, Michowitz Y, Sankhla V, Shah M, Swapna N et al. Neuraxial modulation for refractory ventricular arrhythmias: value of thoracic epidural anesthesia and surgical left cardiac sympathetic denervation. Circulation. 2010;121:2255–62. https://doi.org/10.1161/CIRCULATIONAHA.109.929703
Shen MJ, Chang H, Park H, Akingba AG, Chang P, Zhang Z et al. Low-level vagus nerve stimulation upregulates small conductance calcium-activated potassium channels in the stellate ganglion. Heart Rhythm. 2013;10:910–5. https://doi.org/10.1016/j.hrthm.2013.01.029.
Li D, Wang L, Lee C, Dawson TA, Paterson DJ. Noradrenergic cell specific gene transfer with neuronal nitric oxide synthase reduces cardiac sympathetic neurotransmission in hypertensive rats. Hypertension. 2007;50:69–74. :https://doi.org/10.1161/HYPERTENSIONAHA.107.088591.
Acknowledgments
All individuals who contributed to this publication have been included as authors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The Department of Cardiology of the Leiden University Medical Center received unrestricted research grants from Abbott Vascular, Bayer, Bioventrix, Biotronik, Boston Scientific, Edwards Lifesciences, GE Healthcare, and Medtronic. Pieter van der Bijl has nothing to disclose. Juhani Knuuti received speaker fees from GE Healthcare, Bayer, Lundbeck, and Merck; and fees from AstraZeneca and GE Healthcare for study protocol commenting. Victoria Delgado received speaker fees from Abbott Vascular, MSD, Medtronic, Edwards Lifesciences, and GE Healthcare. Jeroen J. Bax received speaker fees from Abbott Vascular.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Nuclear Cardiology
Rights and permissions
About this article
Cite this article
van der Bijl, P., Knuuti, J., Delgado, V. et al. Cardiac Sympathetic Innervation Imaging with PET Radiotracers. Curr Cardiol Rep 23, 4 (2021). https://doi.org/10.1007/s11886-020-01432-9
Accepted:
Published:
DOI: https://doi.org/10.1007/s11886-020-01432-9