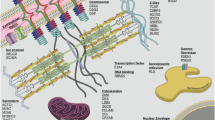
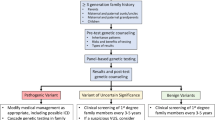
Abstract
Purpose of Review
This review aims to summarize the current knowledge on the genetic background of dilated cardiomyopathy (DCM), with particular attention to the genotype-phenotype correlations and the possible implications for clinical management.
Recent Findings
Next generation sequencing (NGS) has led to the identification of an increasing number of genes and mutations responsible for DCM. This genetic variability is probably related to the extreme heterogeneity of disease manifestation. Important findings have associated mutations of Lamin A/C (LMNA) and Filamin C (FLNC) to poor prognosis and the propensity to cause an arrhythmic phenotype, respectively. However, a deeper understanding of the genotype-phenotype correlation is necessary, because it could have several implications for the clinical management of the patients. Furthermore, the correct interpretation of pathogenicity of mutations and the clinical impact of genetic testing in DCM patients still represent important fields to be implemented.
Summary
A pathogenic gene mutation can be identified in almost 40% of DCM patients. The recent discoveries and future research in the field of genotype-phenotype correlation may lead to a more personalized management of the mutation carriers towards the application of precision medicine in DCM.


Similar content being viewed by others
Abbreviations
- ARVC:
-
Right ventricular arrhythmogenic cardiomyopathy
- DCM:
-
Dilated cardiomyopathy
- DES:
-
Desmin
- DMD:
-
Dystrophin
- DSP:
-
Desmoplakin
- FLNC :
-
Filamin C
- HCM:
-
Hypertrophic cardiomyopathy
- ICD:
-
Implantable cardioverter defibrillator
- LDB3:
-
LIM domain binding 3
- LMNA:
-
Lamin A/C
- LVEF:
-
Left ventricular ejection fraction
- LVRR:
-
Left ventricular reverse remodeling
- MYH7:
-
Beta myosin heavy chain
- MYBPC3:
-
Myosin binding protein C3
- MYPN:
-
Myopalladin
- NEBL:
-
Nebulette
- NEXN:
-
Nexilin F-actin binding protein
- NGS:
-
Next generation sequencing
- OBSL1:
-
Obscurin like 1
- RBM20:
-
RNA-binding motif protein-20
- RCM:
-
Restrictive cardiomyopathy
- SCD:
-
Sudden cardiac death
- SCN5A:
-
Sodium channel protein type 5 subunit alpha
- TNNT2:
-
Cardiac troponin T2
- TPM1:
-
Tropomyosin
- TTN:
-
Titin
- VUS:
-
Variant of uncertain significance
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Mestroni L, Brun F, Spezzacatene A, Sinagra G, Taylor MR. Genetic causes of dilated cardiomyopathy. Prog Pediatr Cardiol. 2014;37:13–8.
Parikh VN, Ashley EA. Next-generation sequencing in cardiovascular disease: present clinical applications and the horizon of precision medicine. Circulation. 2017;135:406–9.
Favalli V, Serio A, Grasso M, Arbustini E. Genetic causes of dilated cardiomyopathy. Heart. 2016;102:2004–14.
•• Haas J, Frese KS, Peil B, et al. Atlas of the clinical genetics of human dilated cardiomyopathy. Eur Heart J. 2015;36:1123–35. This article is the most comprehensive study on the distribution of genes, the number of mutations and mutational burden of patients with DCM.
Sturm AC, Hershberger RE. Genetic testing in cardiovascular medicine: current landscape and future horizons. Curr Opin Cardiol. 2013;28:317–25.
Tayal U, Prasad S, Cook SA. Genetics and genomics of dilated cardiomyopathy and systolic heart failure. Genome Med. 2017;9(1):20. https://doi.org/10.1186/s13073-017-0410-8.
Walsh R, Thomson KL, Ware JS, Funke BH, Woodley J, McGuire KJ, et al. Reassessment of Mendelian gene pathogenicity using 7,855 cardiomyopathy cases and 60,706 reference samples. Genet Med. 2017;19:192–203.
Nouhravesh N, Ahlberg G, Ghouse J, Andreasen C, Svendsen JH, Haunsø S, et al. Analyses of more than 60,000 exomes questions the role of numerous genes previously associated with dilated cardiomyopathy. Mol Genet Genomic Med. 2016;4:617–23.
Richards S, Aziz N, Bale S, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17:405–24.
Li Q, Wang K, McPherson JD, et al. InterVar: clinical interpretation of genetic variants by the 2015 ACMG-AMP Guidelines. Am J Hum Genet 2017:100:267–280. https://doi.org/10.1016/j.ajhg.2017.01.004.
McNally EM, Golbus JR, Puckelwartz MJ. Genetic mutations and mechanisms in dilated cardiomyopathy. J Clin Invest. 2013;123:19–26.
McNally EM, Mestroni L. Dilated cardiomyopathy: genetic determinants and mechanisms. Circ Res. 2017;121:731–48.
Hershberger RE, Norton N, Morales A, Li D, Siegfried JD, Gonzalez-Quintana J. Coding sequence rare variants identified in MYBPC3, MYH6, TPM1, TNNC1, and TNNI3 from 312 patients with familial or idiopathic dilated cardiomyopathy. Circ Cardiovasc Genet. 2010;3:155–61.
Kushner JD, Nauman D, Burgess D, Ludwigsen S, Parks SB, Pantely G, et al. Clinical characteristics of 304 kindreds evaluated for familial dilated cardiomyopathy. J Card Fail. 2006;12:422–9.
Siegfried JD, Morales A, Kushner JD, Burkett E, Cowan J, Mauro AC, et al. Return of genetic results in the familial dilated cardiomyopathy research project. J Genet Couns. 2013;22:164–74.
Hershberger RE, Hedges DJ, Morales A. Dilated cardiomyopathy: the complexity of a diverse genetic architecture. Nat Rev Cardiol. 2013;10:531–47.
Herman DS, Lam L, Taylor MRG, Wang L, Teekakirikul P, Christodoulou D, et al. Truncations of titin causing dilated cardiomyopathy. N Engl J Med. 2012;366:619–28.
Akinrinade O, Koskenvuo JW, Alastalo TP. Prevalence of titin truncating variants in general population. PLoS One. 2015;10(12):e0145284. https://doi.org/10.1371/journal.pone.0145284.
Kamisago M, Sharma SD, DePalma SR, et al. Mutations in sarcomere protein genes as a cause of dilated cardiomyopathy. N Engl J Med. 2000;343(23):1688–96. https://doi.org/10.1056/NEJM200012073432304.
Pugh TJ, Kelly MA, Gowrisankar S, Hynes E, Seidman MA, Baxter SM, et al. The landscape of genetic variation in dilated cardiomyopathy as surveyed by clinical DNA sequencing. Genet Med. 2014;16:601–8.
• Dal Ferro M, Stolfo D, Altinier A, et al. Association between mutation status and left ventricular reverse remodelling in dilated cardiomyopathy. Heart. 2017;103:1704–10. This study provides the important evidence that the response to therapy in terms of LVRR could be influenced by the mutational status (especially, cytoskeleton Z-disk rare variants are associater with lower rate of reverse remodeling).
Begay RL, Graw SL, Sinagra G, Asimaki A, Rowland TJ, Slavov DB, et al. Filamin C truncation mutations are associated with arrhythmogenic dilated cardiomyopathy and changes in the cell–cell adhesion structures. JACC Clin Electrophysiol. 2018;4:504–14.
• Ortiz-Genga MF, Cuenca S, Dal Ferro M, et al. Truncating FLNC mutations are associated with high-risk dilated and arrhythmogenic cardiomyopathies. J Am Coll Cardiol. 2016;68:2440–51. This article is important because it provides the evidence of an arrhythmogenic phenotype caused by FLNC mutations in a relatively large cohort of patients.
Taylor MRG, Slavov D, Ku L, di Lenarda A, Sinagra G, Carniel E, et al. Prevalence of desmin mutations in dilated cardiomyopathy. Circulation. 2007;115:1244–51.
Politano L, Nigro V, Nigro G, Petretta VR, Passamano L, Papparella S, et al. Development of cardiomyopathy in female carriers of Duchenne and Becker muscular dystrophies. J Am Med Assoc. 1996;275:1335–8.
Taylor MRG, Fain PR, Sinagra G, Robinson ML, Robertson AD, Carniel E, et al. Natural history of dilated cardiomyopathy due to lamin A/C gene mutations. J Am Coll Cardiol. 2003;41:771–80.
Elliott P, O’Mahony C, Syrris P, Evans A, Sorensen CR, Sheppard MN, et al. Prevalence of desmosomal protein gene mutations in patients with dilated cardiomyopathy. Circ Cardiovasc Genet. 2010;3:314–22.
Menon SC, Michels VV, Pellikka PA, Ballew JD, Karst ML, Herron KJ, et al. Cardiac troponin T mutation in familial cardiomyopathy with variable remodeling and restrictive physiology. Clin Genet. 2008;74:445–54.
Roncarati R, Viviani Anselmi C, Krawitz P, Lattanzi G, von Kodolitsch Y, Perrot A, et al. Doubly heterozygous LMNA and TTN mutations revealed by exome sequencing in a severe form of dilated cardiomyopathy. Eur J Hum Genet. 2013;21:1105–11.
Wessels MW, Herkert JC, Frohn-Mulder IM, Dalinghaus M, Van Den Wijngaard A, De Krijger RR, et al. Compound heterozygous or homozygous truncating MYBPC3 mutations cause lethal cardiomyopathy with features of noncompaction and septal defects. Eur J Hum Genet. 2015;23:922–8.
Frantz S, Falcao-Pires I, Balligand JL, Bauersachs J, Brutsaert D, Ciccarelli M, et al. The innate immune system in chronic cardiomyopathy: a European Society of Cardiology (ESC) scientific statement from the Working Group on Myocardial Function of the ESC. Eur J Heart Fail. 2018;20:445–59.
Ware JS, Li J, Mazaika E, Yasso CM, DeSouza T, Cappola TP, et al. Shared genetic predisposition in peripartum and dilated cardiomyopathies. N Engl J Med. 2016;374:233–41.
Deshmukh PM, Krishnamani R, Romanyshyn M, Johnson AK, Noti JD. Association of angiotensin converting enzyme gene polymorphism with tachycardia cardiomyopathy. Int J Mol Med. 2004;13:455–8.
Guzzo-merello G, Cobo-marcos M, Gallego-delgado M, Garcia-pavia P, Guzzo-merello G, Cobo-marcos M, et al. Alcoholic cardiomyopathy. World J Cardiol. 2014;6:771–81.
Wasielewski M, Van Spaendonck-Zwarts KY, Westerink NDL, Jongbloed JDH, Postma A, Gietema JA, et al. Potential genetic predisposition for anthracycline-associated cardiomyopathy in families with dilated cardiomyopathy. Open Hear. 2014;1(1):e000116. https://doi.org/10.1136/openhrt-2014-000116.
Ware JS, Amor-salamanca A, Tayal U, et al. Genetic etiology for alcohol-induced cardiac toxicity. J Am Coll Cardiol. 2018;71(20):2293–302. https://doi.org/10.1016/j.jacc.2018.03.462.
Zorzi A, Pelliccia A, Corrado D. Inherited cardiomyopathies and sports participation. Netherlands Hear J. 2018;26:154–65.
Kirchhof P, Fabritz L, Zwiener M, Witt H, Schafers M, Zellerhoff S, et al. Age- and training-dependent development of arrhythmogenic right ventricular cardiomyopathy in heterozygous plakoglobin-deficient mice. Circulation. 2006;114:1799–806.
Hazebroek MR, Moors S, Dennert R, van den Wijngaard A, Krapels I, Hoos M, et al. Prognostic relevance of gene-environment interactions in patients with dilated cardiomyopathy applying the MOGE(S) classification. J Am Coll Cardiol. 2015;66:1313–23.
Charron P, Arad M, Arbustini E, Basso C, Bilinska Z, Elliott P, et al. Genetic counselling and testing in cardiomyopathies: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2010;31:2715–28.
• Merlo M, Cannatà A, Gobbo M, Stolfo D, Elliott PM, Sinagra G. Evolving concepts in dilated cardiomyopathy. Eur J Heart Fail. 2018;20(2):228–39. This review offers a comprehensive survey of emerging issues in the clinical management of DCM, providing where possible practical recommendations.
Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2016:37:2129–2200m.
Ackerman MJ, Priori SG, Willems S, Berul C, Brugada R, Calkins H, et al. HRS/EHRA expert consensus statement on the state of genetic testing for the channelopathies and cardiomyopathies. Europace. 2011;13:1077–109.
Hershberger RE, Lindenfeld J, Mestroni L, Seidman CE, Taylor MRG, Towbin JA, Heart Failure Society of America. Genetic evaluation of cardiomyopathy—a Heart Failure Society of America practice guideline. J Card Fail 2009:15:83–97.
Moretti M, Merlo M, Barbati G, Di Lenarda A, Brun F, Pinamonti B, et al. Prognostic impact of familial screening in dilated cardiomyopathy. Eur J Heart Fail. 2010;12:922–7.
van Spaendonck-Zwarts KY, van den Berg MP, van Tintelen JP. DNA analysis in inherited cardiomyopathies: current status and clinical relevance. Pacing Clin Electrophysiol. 2008;31:S46–9.
Fatkin D, Yeoh T, Hayward CS, Benson V, Sheu A, Richmond Z, et al. Evaluation of left ventricular enlargement as a marker of early disease in familial dilated cardiomyopathy. Circ Cardiovasc Genet. 2011;4:342–8.
• Hasselberg NE, Haland TF, Saberniak J, Brekke PH, Berge KE, Leren TP, et al. Lamin A/C cardiomyopathy: young onset, high penetrance, and frequent need for heart transplantation. Eur Heart J. 2018;39:853–60. This article provides the prevalence and cardiac penetrance of lamin mutation in a large norwegian DCM cohort.
Lakdawala NK, Thune JJ, Colan SD, Cirino AL, Farrohi F, Rivero J, et al. Subtle abnormalities in contractile function are an early manifestation of sarcomere mutations in dilated cardiomyopathy. Circ Cardiovasc Genet. 2012;5:503–10.
Sinagra G, Ferro MD, Merlo M. Lamin A/C cardiomyopathy. Circ Cardiovasc Genet. 2017;10(6):e002004. https://doi.org/10.1161/CIRCGENETICS.117.002004.
Priori SG, Blomström-Lundqvist C, Mazzanti A, et al. ESC guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur Heart J. 2015;2015(36):2793–867.
Gerull B, Gramlich M, Atherton J, McNabb M, Trombitás K, Sasse-Klaassen S, et al. Mutations of TTN, encoding the giant muscle filament titin, cause familial dilated cardiomyopathy. Nat Genet. 2002;30:201–4.
• Verdonschot JAJ, Hazebroek MR, Derks KWJ, et al. Titin cardiomyopathy leads to altered mitochondrial energetics, increased fibrosis and long-term life-threatening arrhythmias. Eur Heart J. 2018;39:864–73. This article shows the structural and metabolic alterations that carachterize heart with titin truncating mutations.
Tayal U, Newsome S, Buchan R, Whiffin N, Halliday B, Lota A, et al. Phenotype and clinical outcomes of titin cardiomyopathy. J Am Coll Cardiol. 2017;70:2264–74.
Begay RL, Graw S, Sinagra G, Merlo M, Slavov D, Gowan K, et al. Role of titin missense variants in dilated cardiomyopathy. J Am Heart Assoc. 2015;4(11):e002645. https://doi.org/10.1161/JAHA.115.002645.
Garcia-Pavia P, Syrris P, Salas C, Evans A, Mirelis JG, Cobo-Marcos M, et al. Desmosomal protein gene mutations in patients with idiopathic dilated cardiomyopathy undergoing cardiac transplantation: a clinicopathological study. Heart. 2011;97:1744–52.
Capetanaki Y, Papathanasiou S, Diokmetzidou A, Vatsellas G, Tsikitis M. Desmin related disease: a matter of cell survival failure. Curr Opin Cell Biol. 2015;32:113–20.
Arbustini E, Pasotti M, Pilotto A, Pellegrini C, Grasso M, Previtali S, et al. Desmin accumulation restrictive cardiomyopathy and atrioventricular block associated with desmin gene defects. Eur J Heart Fail. 2006;8:477–83.
Schmitt JP, Kamisago M, Asahi M, Li GH, Ahmad F, Mende U, et al. Dilated cardiomyopathy and heart failure caused by a mutation in phospholamban. Science. 2003;299:1410–3.
Fish M, Shaboodien G, Kraus S, Sliwa K, Seidman CE, Burke MA, et al. Mutation analysis of the phospholamban gene in 315 South Africans with dilated, hypertrophic, peripartum and arrhythmogenic right ventricular cardiomyopathies. Sci Rep. 2016;6:22235.
Haghighi K, Kolokathis F, Gramolini AO, Waggoner JR, Pater L, Lynch RA, et al. A mutation in the human phospholamban gene, deleting arginine 14, results in lethal, hereditary cardiomyopathy. Proc Natl Acad Sci. 2006;103:1388–93.
van der Zwaag PA, van Rijsingen IAW, Asimaki A, Jongbloed JDH, van Veldhuisen DJ, Wiesfeld ACP, et al. Phospholamban R14del mutation in patients diagnosed with dilated cardiomyopathy or arrhythmogenic right ventricular cardiomyopathy: evidence supporting the concept of arrhythmogenic cardiomyopathy. Eur J Heart Fail. 2012;14:1199–207.
McNair WP, Sinagra G, Taylor MRG, di Lenarda A, Ferguson DA, Salcedo EE, et al. SCN5A mutations associate with arrhythmic dilated cardiomyopathy and commonly localize to the voltage-sensing mechanism. J Am Coll Cardiol. 2011;57:2160–8.
Li D, Morales A, Gonzalez-Quintana J, Norton N, Siegfried JD, Hofmeyer M, et al. Identification of novel mutations in RBM20 in patients with dilated cardiomyopathy. Clin Transl Sci. 2010;3(3):90–7. https://doi.org/10.1111/j.1752-8062.2010.00198.x.
Guo W, Schafer S, Greaser ML, Radke MH, Liss M, Govindarajan T, et al. RBM20, a gene for hereditary cardiomyopathy, regulates titin splicing. Nat Med. 2012;18(5):766–73. https://doi.org/10.1038/nm.2693.
Benedetti S, Menditto I, Degano M, Rodolico C, Merlini L, D'Amico A, et al. Phenotypic clustering of lamin A/C mutations in neuromuscular patients. Neurology. 2007;69:1285–92.
Chen L, Lee L, Kudlow BA, Dos Santos HG, Sletvold O, Shafeghati Y, et al. LMNA mutations in atypical Werner’s syndrome. Lancet. 2003;362:440–5.
Van Rijsingen IAW, Arbustini E, Elliott PM, et al. Risk factors for malignant ventricular arrhythmias in lamin A/C mutation carriers: a European cohort study. J Am Coll Cardiol. 2012;59:493–500.
van Rijsingen IAW, Nannenberg EA, Arbustini E, Elliott PM, Mogensen J, Hermans-van Ast JF, et al. Gender-specific differences in major cardiac events and mortality in lamin A/C mutation carriers. Eur J Heart Fail. 2013;15:376–84.
Pasotti M, Klersy C, Pilotto A, Marziliano N, Rapezzi C, Serio A, et al. Long-term outcome and risk stratification in dilated cardiolaminopathies. J Am Coll Cardiol. 2008;52:1250–60.
Burke MA, Cook SA, Seidman JG, Seidman CE. Clinical and mechanistic insights into the genetics of cardiomyopathy. J Am Coll Cardiol. 2016;68(25):2871–86. https://doi.org/10.1016/j.jacc.2016.08.079.
Vorgerd M, van der Ven PFM, Bruchertseifer V, Löwe T, Kley RA, Schröder R, et al. A mutation in the dimerization domain of filamin C causes a novel type of autosomal dominant myofibrillar myopathy. Am J Hum Genet. 2005;77:297–304.
Kley RA, Hellenbroich Y, van der Ven PFM, Furst DO, Huebner A, Bruchertseifer V, et al. Clinical and morphological phenotype of the filamin myopathy: a study of 31 German patients. Brain. 2007;130:3250–64.
Valdés-Mas R, Gutiérrez-Fernández A, Gómez J, Coto E, Astudillo A, Puente DA, et al. Mutations in filamin C cause a new form of familial hypertrophic cardiomyopathy. Nat Commun. 2014;5:5326. https://doi.org/10.1038/ncomms6326.
Brodehl A, Ferrier RA, Hamilton SJ, Greenway SC, Brundler MA, Yu W, et al. Mutations in FLNC are associated with familial restrictive cardiomyopathy. Hum Mutat. 2016;37(3):269–79. https://doi.org/10.1002/humu.22942.
Stossel TP, Condeelis J, Cooley L, Hartwig JH, Noegel A, Schleicher M, et al. Filamins as integrators of cell mechanics and signalling. Nat Rev Mol Cell Biol. 2001;2:138–45.
Zhou A-X, Hartwig JH, Akyürek LM. Filamins in cell signaling, transcription and organ development. Trends Cell Biol. 2010;20:113–23.
Corrado D, Basso C, Judge DP. Arrhythmogenic cardiomyopathy. Circ Res. 2017;121:784–802.
LeWinter MM, Granzier HL. Cardiac titin and heart disease. J Cardiovasc Pharmacol. 2014;63:207–12.
Hastings R, De Villiers CP, Hooper C, et al. Combination of whole genome sequencing, linkage, and functional studies implicates a missense mutation in titin as a cause of autosomal dominant cardiomyopathy with features of left ventricular noncompaction. Circ Cardiovasc Genet. 2016;9:426–35.
Sarantitis I, Papanastasopoulos P, Manousi M, Baikoussis NG, Apostolakis E. The cytoskeleton of the cardiac muscle cell. Hell J Cardiol. 2012;53:367–79.
Li D, Tapscoft T, Gonzalez O, Burch PE, Quiñones MA, Zoghbi WA, et al. Desmin mutation responsible for idiopathic dilated cardiomyopathy. Circulation. 1999;100:461–4. https://doi.org/10.1161/01.CIR.100.5.461.
Liu G-S, Morales A, Vafiadaki E, Lam CK, Cai W-F, Haghighi K, et al. A novel human R25C-phospholamban mutation is associated with super-inhibition of calcium cycling and ventricular arrhythmia. Cardiovasc Res. 2015;107:164–74.
te Rijdt WP, van Tintelen JP, Vink A, van der Wal AC, de Boer RA, van den Berg MP, et al. Phospholamban p.Arg14del cardiomyopathy is characterized by phospholamban aggregates, aggresomes, and autophagic degradation. Histopathology. 2016;69:542–50.
Dewitt MM, Macleod HM, Soliven B, Mcnally EM, Chicago I. Phospholamban R14 deletion results in late-onset, mild, hereditary dilated cardiomyopathy. J Am Coll Cardiol. 2006;48(7):1396–8. https://doi.org/10.1016/j.jacc.2006.07.016.
Truszkowska GT, Bilińska ZT, Kosińska J, Śleszycka J, Rydzanicz M, Sobieszczańska-Małek M, et al. A study in polish patients with cardiomyopathy emphasizes pathogenicity of phospholamban (PLN) mutations at amino acid position 9 and low penetrance of heterozygous null PLN mutations. BMC Med Genet. 2015;16:21. https://doi.org/10.1186/s12881-015-0167-0.
Liang P, Liu W, Li C, Tao W, Li L, Hu D. Genetic analysis of Brugada syndrome and congenital long-QT syndrome type 3 in the Chinese. J Cardiovasc Dis Res. 2010;1:69–74.
Liang P, Sallam K, Wu H, Li Y, Itzhaki I, Garg P, et al. Patient-specific and genome-edited induced pluripotent stem cell–derived cardiomyocytes elucidate single-cell phenotype of Brugada syndrome. J Am Coll Cardiol. 2016;68(19):2086–96. https://doi.org/10.1016/j.jacc.2016.07.779.
Maatz H, Jens M, Liss M, Schafer S, Heinig M, Kirchner M, et al. RNA-binding protein RBM20 represses splicing to orchestrate cardiac pre-mRNA processing. J Clin Invest. 2014;124(8):3419–30. https://doi.org/10.1172/JCI74523.
Cohn JN, Ferrari R, Sharpe N. Cardiac remodeling—concepts and clinical implications: a consensus paper from an international forum on cardiac remodeling. J Am Coll Cardiol. 2000;35:569–82.
Aleksova A, Sabbadini G, Merlo M, Pinamonti B, Barbati G, Zecchin M, et al. Natural history of dilated cardiomyopathy: from asymptomatic left ventricular dysfunction to heart failure—a subgroup analysis from the Trieste Cardiomyopathy Registry. J Cardiovasc Med. 2009;10:699–705.
Merlo M, Caiffa T, Gobbo M, Adamo L, Sinagra G. Reverse remodeling in dilated cardiomyopathy: insights and future perspectives. IJC Hear Vasc. 2018;18:52–7.
Merlo M, Pivetta A, Pinamonti B, Stolfo D, Zecchin M, Barbati G, et al. Long-term prognostic impact of therapeutic strategies in patients with idiopathic dilated cardiomyopathy: changing mortality over the last 30 years. Eur J Heart Fail. 2014;16:317–24.
Bowles NE, Bowles KR, Towbin JA. The "final common pathway" hypothesis and inherited cardiovascular disease. Herz. 2000;25:168–75.
Towbin JA, Lorts A, Cincinnati O. Arrhythmias and dilated cardiomyopathy common pathogenetic pathways?*. J Am Coll Cardiol. 2011;57:2169–71.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Alessia Paldino, Giulia De Angelis, Marco Merlo, Marta Gigli, Matteo Dal Ferro, Giovanni Maria Severini, Luisa Mestroni and Gianfranco Sinagra declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Myocardial Disease
Rights and permissions
About this article
Cite this article
Paldino, A., De Angelis, G., Merlo, M. et al. Genetics of Dilated Cardiomyopathy: Clinical Implications. Curr Cardiol Rep 20, 83 (2018). https://doi.org/10.1007/s11886-018-1030-7
Published:
DOI: https://doi.org/10.1007/s11886-018-1030-7