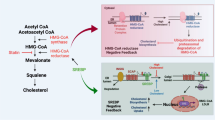
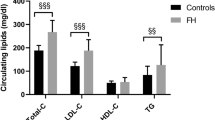
Abstract
Purpose of Review
We seek to establish whether high-density lipoprotein HDL metabolism and reverse cholesterol transport (RCT) impairment is an intrinsic feature of familial hypercholesterolemia (FH).
Recent Findings
RCT from macrophages (m-RCT), a vascular cell type of major influence on atherosclerosis, is impaired in FH due to defective low-density lipoprotein receptor (LDLR) function via both the HDL- and LDL-mediated pathways. Potential mechanisms include impaired HDL metabolism, which is linked to increased LDL levels, as well as the increased transport of cellular unesterified cholesterol to LDL, which presents a defective catabolism.
Summary
RCT dysfunction is consistently associated with mutation-positive FH linked to decreased HDL levels as well as impaired HDL remodeling and LDLR function. It remains to be explored whether these alterations are also present in less well-characterized forms of FH, such as cases with no identified mutations, and whether they are fully corrected by current standard treatments.

Similar content being viewed by others
Abbreviations
- ABC:
-
ATP-binding cassette transporter
- APO:
-
Apolipoprotein
- CE:
-
Cholesterol ester
- CEC:
-
Cholesterol efflux capacity
- CETP:
-
Cholesteryl ester transfer protein
- CVD:
-
Cardiovascular disease
- FH:
-
Familial hypercholesterolemia
- HDL:
-
High-density lipoprotein
- HDL-c:
-
HDL cholesterol
- LCAT:
-
Lecithin:cholesterol acyltransferase
- LDL:
-
Low-density lipoprotein
- LDL-c:
-
LDL cholesterol
- LDLR:
-
LDL receptor
- LXR:
-
Liver X receptor
- miRNA:
-
Micro RNA
- m-RCT:
-
Macrophage-specific reverse cholesterol transport
- PCSK9:
-
Proprotein convertase subtilisin/kexin type 9
- PLTP:
-
Phospholipid transfer protein
- RCT:
-
Reverse cholesterol transport
- SR-BI:
-
Scavenger receptor BI
- SREBP:
-
Sterol response element-binding protein
- TICE:
-
Transintestinal cholesterol excretion
- UC:
-
Unesterified cholesterol
- VLDL:
-
Very low-density lipoprotein
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Nordestgaard BG, Chapman MJ, Humphries SE, et al. Familial hypercholesterolaemia is underdiagnosed and undertreated in the general population: guidance for clinicians to prevent coronary heart disease: consensus statement of the European Atherosclerosis Society. Eur Heart J. 2013;34(45):3478–3490a.
Talmud PJ, Shah S, Whittall R, Futema M, Howard P, Cooper JA, et al. Use of low-density lipoprotein cholesterol gene score to distinguish patients with polygenic and monogenic familial hypercholesterolaemia: a case-control study. Lancet. 2013;381(9874):1293–301.
Martin-Campos JM, Ruiz-Nogales S, Ibarretxe D, et al. Polygenic markers in patients diagnosed of autosomal dominant hypercholesterolemia in catalonia: distribution of weighted LDL-c-raising SNP scores and refinement of variant selection. Biomedicines. 2020;8(9):353. In line with previous findings of different groups, the increased LDL genetic score based on selected SNPs in mutation-negative FH patients suggest the existence of polygenic forms of the disease.
Kontush A. HDL and reverse remnant-cholesterol transport (RRT): Relevance to cardiovascular disease. Trends Mol Med. 2020;26(12):1086–100. This review proposes a potential novel pathway that explains the U-shaped relationship between plasma HDL-C levels and cardiovascular disease via the impairment of the transfer of unesterified cholesterol from triglyceride-rich lipoproteins to HDL.
Rosales C, Gillard BK, Xu B, Gotto AM Jr, Pownall HJ. Revisiting reverse cholesterol transport in the context of high-density lipoprotein free cholesterol bioavailability. Methodist Debakey Cardiovasc J. 2019;15(1):47–54. This review hypothesizes that an increased bioavailability of unesterified cholesterol in dysfunctional HDL would promote the excess transfer of this molecule to cells, thus constituting a potential proatherogenic mechanism.
Jansen AC, van Aalst-Cohen ES, Tanck MW, et al. The contribution of classical risk factors to cardiovascular disease in familial hypercholesterolaemia: data in 2400 patients. J Intern Med. 2004;256(6):482–90.
van Aalst-Cohen ES, Jansen AC, Boekholdt SM, et al. Genetic determinants of plasma HDL-cholesterol levels in familial hypercholesterolemia. Eur J Hum Genet. 2005;13(10):1137–42.
Inazu A, Koizumi J, Mabuchi H, Kajinami K, Takeda R. Enhanced cholesteryl ester transfer protein activities and abnormalities of high density lipoproteins in familial hypercholesterolemia. Horm Metab Res. 1992;24(6):284–8.
Bellanger N, Orsoni A, Julia Z, Fournier N, Frisdal E, Duchene E, et al. Atheroprotective reverse cholesterol transport pathway is defective in familial hypercholesterolemia. Arterioscler Thromb Vasc Biol. 2011;31(7):1675–81.
Badimon L, Padro T, Cubedo J. Protein changes in non-LDL-lipoproteins in familial hypercholesterolemia: implications in cardiovascular disease manifestation and outcome. Curr Opin Lipidol. 2017;28(5):427–33. This review focuses on the identification of HDL protein changes that might contribute to the increased cardiovascular risk of familial hypercholesterolemia.
Hogue JC, Lamarche B, Gaudet D, Tremblay AJ, Després JP, Bergeron J, et al. Association of heterozygous familial hypercholesterolemia with smaller HDL particle size. Atherosclerosis. 2007;190(2):429–35.
Hussein H, Saheb S, Couturier M, Atassi M, Orsoni A, Carrié A, et al. Small, dense high-density lipoprotein 3 particles exhibit defective antioxidative and anti-inflammatory function in familial hypercholesterolemia: Partial correction by low-density lipoprotein apheresis. J Clin Lipidol. 2016;10(1):124–33.
Koizumi J, Inazu A, Fujita H, et al. Removal of apolipoprotein E-enriched high density lipoprotein by LDL-apheresis in familial hypercholesterolaemia: a possible activation of the reverse cholesterol transport system. Atherosclerosis. 1988;74(1-2):1–8.
Cedo L, Plana N, Metso J, et al. Altered HDL remodeling and functionality in familial hypercholesterolemia. J Am Coll Cardiol. 2018;71(4):466–8. Short communication showing impairment in lipid transfer proteins and enzymes regulating HDL remodeling and cholesterol efflux in untreated heterozygous patients with an LDLR identified mutation.
Miida T, Nakamura Y, Okada M. Development of coronary atherosclerosis in asymptomatic heterozygous patients with familial hypercholesterolemia. J Cardiol. 1996;28(2):71–7.
Frenais R, Ouguerram K, Maugeais C, et al. Apolipoprotein A-I kinetics in heterozygous familial hypercholesterolemia: a stable isotope study. J Lipid Res. 1999;40(8):1506–11.
Schaefer JR, Rader DJ, Ikewaki K, Fairwell T, Zech LA, Kindt MR, et al. In vivo metabolism of apolipoprotein A-I in a patient with homozygous familial hypercholesterolemia. Arterioscler Thromb. 1992;12(7):843–8.
Gibson JC, Goldberg RB, Rubinstein A, Ginsberg HN, Brown WV, Baker S, et al. Plasma lipoprotein distribution of apolipoprotein E in familial hypercholesterolemia. Arteriosclerosis. 1987;7(4):401–7.
Miltiadous G, Cariolou MA, Elisaf M. HDL cholesterol levels in patients with molecularly defined familial hypercholesterolemia. Ann Clin Lab Sci. 2002;32(1):50–4.
Cubedo J, Padro T, Alonso R, Mata P, Badimon L. ApoL1 levels in high density lipoprotein and cardiovascular event presentation in patients with familial hypercholesterolemia. J Lipid Res. 2016;57(6):1059–73.
Swertfeger DK, Rebholz S, Li H, Shah AS, Davidson WS, Lu LJ. Feasibility of a plasma bioassay to assess oxidative protection of low-density lipoproteins by high-density lipoproteins. J Clin Lipidol. 2018;12(6):1539–48.
Cedo L, Metso J, Santos D, et al. LDL receptor regulates the reverse transport of macrophage-derived unesterified cholesterol via concerted action of the HDL-LDL axis: insight from mouse models. Circ Res. 2020;127(6):778–92. In vivo demonstration of impaired m-RCT in mouse models of familial hypercholesterolemia due to major LDLR dysfunction, but not in models with a similar phenotype but conserved LDLR capacity, such as the apoB100 transgenic mice.
Lakomy D, Rebe C, Sberna AL, et al. Liver X receptor-mediated induction of cholesteryl ester transfer protein expression is selectively impaired in inflammatory macrophages. Arterioscler Thromb Vasc Biol. 2009;29(11):1923–9.
Laffitte BA, Joseph SB, Chen M, Castrillo A, Repa J, Wilpitz D, et al. The phospholipid transfer protein gene is a liver X receptor target expressed by macrophages in atherosclerotic lesions. Mol Cell Biol. 2003;23(6):2182–91.
Rohatgi A, Khera A, Berry JD, Givens EG, Ayers CR, Wedin KE, et al. HDL cholesterol efflux capacity and incident cardiovascular events. N Engl J Med. 2014;371(25):2383–93.
Saleheen D, Scott R, Javad S, Zhao W, Rodrigues A, Picataggi A, et al. Association of HDL cholesterol efflux capacity with incident coronary heart disease events: a prospective case-control study. Lancet Diabetes Endocrinol. 2015;3(7):507–13.
Ottestad IO, Halvorsen B, Balstad TR, Otterdal K, Borge GI, Brosstad F, et al. Triglyceride-rich HDL3 from patients with familial hypercholesterolemia are less able to inhibit cytokine release or to promote cholesterol efflux. J Nutr. 2006;136(4):877–81.
Ogura M, Hori M, Harada-Shiba M. Association between cholesterol efflux capacity and atherosclerotic cardiovascular disease in patients with familial hypercholesterolemia. Arterioscler Thromb Vasc Biol. 2016;36(1):181–8.
Soria-Florido MT, Schroder H, Grau M, Fito M, Lassale C. High density lipoprotein functionality and cardiovascular events and mortality: a systematic review and meta-analysis. Atherosclerosis. 2020;302:36–42. Systematic review and meta-analysis showing that although increased HDL cholesterol efflux capacity and antioxidant/anti-inflammatory capacities are associated with a lower risk of cardiovascular disease, there is a need of larger prospective studies with predefined standardized assays and outcomes.
Guerin M, Silvain J, Gall J, Darabi M, Berthet M, Frisdal E, et al. Association of serum cholesterol efflux capacity with mortality in patients with ST-segment elevation myocardial infarction. J Am Coll Cardiol. 2018;72(25):3259–69.
von Eckardstein A. LDL contributes to reverse cholesterol transport. Circ Res. 2020;127(6):793–5. Insightful editorial comment on the role of LDL on CEC measurements and on the potential significance of unesterified cholesterol transfer from HDL to LDL in atherogenesis.
Guerin M, Dolphin PJ, Chapman MJ. Preferential cholesteryl ester acceptors among the LDL subspecies of subjects with familial hypercholesterolemia. Arterioscler Thromb. 1994;14(5):679–85.
Feng M, Darabi M, Tubeuf E, et al. Free cholesterol transfer to high-density lipoprotein (HDL) upon triglyceride lipolysis underlies the U-shape relationship between HDL-cholesterol and cardiovascular disease. Eur J Prev Cardiol. 2020;27(15):1606–16. Study revealing that unesterified cholesterol transfer to HDL is linked to triglyceride-rich lipoprotein lipolysis and parallels the U-shape relationship between HDL-C and cardiovascular disease.
Sankaranarayanan S, de la Llera-Moya M, Drazul-Schrader D, Phillips MC, Kellner-Weibel G, Rothblat GH. Serum albumin acts as a shuttle to enhance cholesterol efflux from cells. J Lipid Res. 2013;54(3):671–6.
Mosig S, Rennert K, Buttner P, et al. Monocytes of patients with familial hypercholesterolemia show alterations in cholesterol metabolism. BMC Med Genet. 2008;1:60.
Canfran-Duque A, Lin CS, Goedeke L, Suarez Y, Fernandez-Hernando C. Micro-RNAs and high-density lipoprotein metabolism. Arterioscler Thromb Vasc Biol. 2016;36(6):1076–84.
Rottiers V, Naar AM. MicroRNAs in metabolism and metabolic disorders. Nat Rev Mol Cell Biol. 2012;13(4):239–50.
Vickers KC, Palmisano BT, Shoucri BM, Shamburek RD, Remaley AT. MicroRNAs are transported in plasma and delivered to recipient cells by high-density lipoproteins. Nat Cell Biol. 2011;13(4):423–33.
Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120(1):15–20.
Friedman RC, Farh KK, Burge CB, Bartel DP. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009;19(1):92–105.
Wang L, Jia XJ, Jiang HJ, du Y, Yang F, Si SY, et al. MicroRNAs 185, 96, and 223 repress selective high-density lipoprotein cholesterol uptake through posttranscriptional inhibition. Mol Cell Biol. 2013;33(10):1956–64.
Vickers KC, Landstreet SR, Levin MG, Shoucri BM, Toth CL, Taylor RC, et al. MicroRNA-223 coordinates cholesterol homeostasis. Proc Natl Acad Sci U S A. 2014;111(40):14518–23.
Martino F, Carlomosti F, Avitabile D, Persico L, Picozza M, Barillà F, et al. Circulating miR-33a and miR-33b are up-regulated in familial hypercholesterolaemia in paediatric age. Clin Sci (Lond). 2015;129(11):963–72.
D'Agostino M, Martino F, Sileno S, et al. Circulating miR-200c is up-regulated in paediatric patients with familial hypercholesterolaemia and correlates with miR-33a/b levels: implication of a ZEB1-dependent mechanism. Clin Sci (Lond). 2017;131(18):2397–408. Study showing that circulating miR-200c is upregulated in pediatric FH, probably due to oxidative stress and inflammation and via a miR-33a/b-ZEB1-dependent mechanism.
Rayner KJ, Suarez Y, Davalos A, Parathath S, Fitzgerald ML, Tamehiro N, et al. MiR-33 contributes to the regulation of cholesterol homeostasis. Science. 2010;328(5985):1570–3.
Najafi-Shoushtari SH, Kristo F, Li Y, Shioda T, Cohen DE, Gerszten RE, et al. MicroRNA-33 and the SREBP host genes cooperate to control cholesterol homeostasis. Science. 2010;328(5985):1566–9.
Marquart TJ, Allen RM, Ory DS. Baldan A: miR-33 links SREBP-2 induction to repression of sterol transporters. Proc Natl Acad Sci U S A. 2010;107(27):12228–32.
Rinninger F, Heine M, Singaraja R, Hayden M, Brundert M, Ramakrishnan R, et al. High density lipoprotein metabolism in low density lipoprotein receptor-deficient mice. J Lipid Res. 2014;55(9):1914–24.
Tanigawa H, Billheimer JT, Tohyama J, Zhang Y, Rothblat G, Rader DJ. Expression of cholesteryl ester transfer protein in mice promotes macrophage reverse cholesterol transport. Circulation. 2007;116(11):1267–73.
Le May C, Berger JM, Lespine A, et al. Transintestinal cholesterol excretion is an active metabolic process modulated by PCSK9 and statin involving ABCB1. Arterioscler Thromb Vasc Biol. 2013;33(7):1484–93.
Orsoni A, Saheb S, Levels JH, et al. LDL-apheresis depletes apoE-HDL and pre-beta1-HDL in familial hypercholesterolemia: relevance to atheroprotection. J Lipid Res. 2011;52(12):2304–13.
Orsoni A, Villard EF, Bruckert E, Robillard P, Carrie A, Bonnefont-Rousselot D, et al. Impact of LDL apheresis on atheroprotective reverse cholesterol transport pathway in familial hypercholesterolemia. J Lipid Res. 2012;53(4):767–75.
Tao H, Huang J, Yancey PG, et al. Scavenging of reactive dicarbonyls with 2-hydroxybenzylamine reduces atherosclerosis in hypercholesterolemic Ldlr(-/-) mice. Nat Commun. 2020;11(1):4084. Treatment of FH mice with reactive dicarbonyl scavengers reduced HDL oxidative modification and increased HDL cholesterol efflux capacity; HDL from subjects with FH had increased MDA-apoAI adducts and defective cholesterol efflux capacity.
Yahya R, Favari E, Calabresi L, Verhoeven AJM, Zimetti F, Adorni MP, et al. Lomitapide affects HDL composition and function. Atherosclerosis. 2016;251:15–8.
Lappegard KT, Kjellmo CA, Ljunggren S, et al. Lipoprotein apheresis affects lipoprotein particle subclasses more efficiently compared to the PCSK9 inhibitor evolocumab, a pilot study. Transfus Apher Sci. 2018;57(1):91–6.
Adorni MP, Cipollari E, Favari E, Zanotti I, Zimetti F, Corsini A, et al. Inhibitory effect of PCSK9 on Abca1 protein expression and cholesterol efflux in macrophages. Atherosclerosis. 2017;256:1–6.
Christensen JJ, Ulven SM, Retterstol K, et al. Comprehensive lipid and metabolite profiling of children with and without familial hypercholesterolemia: a cross-sectional study. Atherosclerosis. 2017;266:48–57. Interesting study showing that the NMR plasma analysis of children uncovers major changes in FH HDL.
Shimizu T, Miura S, Tanigawa H, Kuwano T, Zhang B, Uehara Y, et al. Rosuvastatin activates ATP-binding cassette transporter A1-dependent efflux ex vivo and promotes reverse cholesterol transport in macrophage cells in mice fed a high-fat diet. Arterioscler Thromb Vasc Biol. 2014;34(10):2246–53.
Tardy C, Goffinet M, Boubekeur N, Ackermann R, Sy G, Bluteau A, et al. CER-001, a HDL-mimetic, stimulates the reverse lipid transport and atherosclerosis regression in high cholesterol diet-fed LDL-receptor deficient mice. Atherosclerosis. 2014;232(1):110–8.
Hovingh GK, Smits LP, Stefanutti C, Soran H, Kwok S, de Graaf J, et al. The effect of an apolipoprotein A-I-containing high-density lipoprotein-mimetic particle (CER-001) on carotid artery wall thickness in patients with homozygous familial hypercholesterolemia: The Modifying Orphan Disease Evaluation (MODE) study. Am Heart J. 2015;169(5):736–42 e731.
Zheng KH, Kaiser Y, van Olden CC, Santos RD, Dasseux JL, Genest J, et al. No benefit of HDL mimetic CER-001 on carotid atherosclerosis in patients with genetically determined very low HDL levels. Atherosclerosis. 2020;311:13–9.
Funding
This article was partly funded by the Instituto de Salud Carlos III and FEDER “Una manera de hacer Europa” grants PI18/00164 (to F.B.-V.), PI17/00232 (to J. J.), and PI19/00136 (to J.C.E-G), and Miguel Servet Type 2 contract (CPII18/00004 to J.J.); grants 12/C/2015 (to F.B-V) and 201602.31 (to J.J.) from La Fundació la Marató TV3; Ministerio de Ciencia, Innovación y Universidades, Subprograma Ramón y Cajal (RyC-20172879 to N.R.); and Red de Investigación “Enfermedades Metabólicas y Cáncer” (RED2018-102799-T to J.J). CIBERDEM is an Instituto de Salud Carlos III project.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Vascular Biology
Rights and permissions
About this article
Cite this article
Escolà-Gil, J.C., Rotllan, N., Julve, J. et al. Reverse Cholesterol Transport Dysfunction Is a Feature of Familial Hypercholesterolemia. Curr Atheroscler Rep 23, 29 (2021). https://doi.org/10.1007/s11883-021-00928-1
Accepted:
Published:
DOI: https://doi.org/10.1007/s11883-021-00928-1