Abstract
Purpose of the Review
Coronavirus disease 2019 (COVID-19), a new infectious disease caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), has reached a pandemic status. Although SARSCoV-2 causes primarily respiratory problems, concurrent cardiac injury cannot be ignored since it may be an independent predictor for adverse outcomes. To resolve these issues, we aim to summarize the prevalence and its underlying mechanisms of acute cardiac injury in the setting of SARS-CoV-2 infection.
Recent Findings
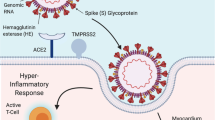
The main clinical manifestation of SARS-CoV-2 infection is pneumonia, cardiovascular complications have also been identified in the earliest reported cases from Wuhan, the epicenter of the outbreak. Given the SARS-CoV-2 likely uses the angiotensin-converting enzyme-2 (ACE2) receptors as its host receptor, ACE2-related signaling pathways may play a key role in mediating myocardial injury.
Summary
SARS-CoV-2 infection related acute cardiac injury cannot be ignored, and its underlying mechanisms remain speculated. We would suggest that health professionals investigate cardiac function as part of the routine care.
Similar content being viewed by others
The emergence of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV2) epidemic is a major global public health concern. Following the initial outbreak as first reported in Wuhan, Hubei Province of the People’s Republic of China [1], person-to-person transmission has already occurred in Mainland China [2,3,4], and in other parts of the world [5].While SARS-CoV2 is primarily a respiratory infection, cardiac injury induced by the virus cannot be neglected. A recent study of 41 patients reported a 12% (5/41) incidence of virus-related acute cardiac injury and 33% (13/40) of elevated serum cardiac biomarker levels [6]. Another study reported a similar rate of 15% of the patients showing elevated cardiac biomarkers [7]. Wang and colleagues found that 7% (10/138) of patients with 2019-nCoV infections developed acute cardiac injury, of whom eight were admitted to the intensive care unit (ICU), accounting for 22% of the total number of ICU patients [8].The same study also reported 17% (23/138) of the patients developed cardiac arrhythmias. Together, data from these studies illustrate that the 2019-nCoV infection results in varying degrees of myocardial damage, which may be explained by adverse outcomes observed.
The prognosis of those with 2019-nCoV is determined by different factors. Approximately half of those infected had multiple comorbidities, mainly cardiovascular and cerebrovascular diseases [8]. Huang et al. pointed out that at baseline, the ICU care group have a higher frequency of underlying diseases, such as cardiovascular disease (23% vs. 11%) compared with those who did not need ICU care [6]. Similarly, Wang et al. reported higher frequency of hypertension (58% vs. 22%) and cardiovascular disease (26% vs. 11%) [8]. Mechanistically, similar to the severe acute respiratory syndrome (SARS)-CoV [9], the 2019-nCoV likely uses the angiotensin-converting enzyme-2 (ACE2) receptors as its host receptor. Given that ACE2 receptors are widely expressed in the cardiovascular system, theoretically these sites are potential targets for 2019-nCoV infection. Indeed, ACE2-related signaling pathways may play a key role in mediating myocardial injury. Cytokine storms, which are associated with an imbalance between types 1 and 2 helper T cells, may explain the severe phenotype of the disease and mediate myocardial damage. Prognosis may be improved by therapies that reduce the inflammatory response. Recent work has found that transplantation of ACE2− mesenchymal stem cells by the intravenous route led to improvement in symptoms and pulmonary function of 2019-nCoV patients [10]. As noted by the accompanying editorial [11], the mechanisms likely involve reduced overactivation of the immune system, as reflected by lower tumor necrosis factor-α and C-reactive protein levels, increased peripheral lymphocytes and CD14+CD11c+CD11bmid regulatory dendritic cells, and disappearance of the cytokine-secreting immune cells CXCR3+CD4+ T cells, CXCR3+CD8+ T cells, and CXCR3+ natural killer cells [10].
In conclusion, the clinical course of patients harboring the 2019-nCoV infection remains to be fully elucidated. We would suggest that health professionals investigate cardiac function as part of the routine care, and the data may shed light on whether myocardial damage is an independent predictor of adverse outcomes.
References
Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. A novel coronavirus from patients with pneumonia in China, 2019. New Eng J Med. 2020;382(8):727–33.
Wang C, Horby PW, Hayden FG, Gao GF. A novel coronavirus outbreak of global health concern. Lancet. 2020;395(10223):470–3.
Chan JF-W, Yuan S, Kok K-H, To KK-W, Chu H, Yang J, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395(10223):514–23.
Pan X, Chen D, Xia Y, Wu X, Li T, Ou X, et al. Asymptomatic cases in a family cluster with SARS-CoV-2 infection. Lancet Infect Dis. 2020. https://doi.org/10.1016/S1473-3099(20)30114-6.
Liu Y-C, Liao C-H, Chang C-F, Chou C-C, Lin Y-R. A locally transmitted case of SARS-CoV-2 infection in Taiwan. New Eng J Med. 2020. https://doi.org/10.1056/NEJMc2001573.
Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506.
Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–13.
Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020. https://doi.org/10.1001/jama.2020.1585.
Zhao Y, Zhao Z, Wang Y, Zhou Y, Ma Y, Zuo W. Single-cell RNA expression profiling of ACE2, the putative receptor of Wuhan 2019-nCov. bioRxiv. 2020. https://doi.org/10.1101/2020.01.26.919985.
Leng Z, Zhu R, Hou W, Feng Y, Yang Y, Han Q, et al. Transplantation of ACE2-mesenchymal stem cells improves the outcome of patients with COVID-19 pneumonia. Aging Dis. 2020;11(2):216–28.
Shetty A-K. Mesenchymal stem cell infusion shows promise for combating coronavirus (COVID-19)-induced pneumonia[J]. Aging Dis. 2020. https://doi.org/10.14336/AD.2020.0301.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, Y., Roever, L., Tse, G. et al. 2019-Novel Coronavirus-Related Acute Cardiac Injury Cannot Be Ignored. Curr Atheroscler Rep 22, 14 (2020). https://doi.org/10.1007/s11883-020-00842-y
Published:
DOI: https://doi.org/10.1007/s11883-020-00842-y