Abstract
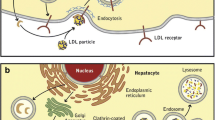
The link between proprotein convertase subtilisin/kexin type 9 (PCSK9) and cholesterol metabolism was established only in 2003 when genetic mapping and positional cloning in patients with autosomal dominant hypercholesterolemia in which linkage to the loci coding for the LDL receptor and apolipoprotein B had been excluded identified the genetic defect missense as mutations in PCSK9, a protein/enzyme previously unknown to be related to lipid metabolism. Laboratory-based investigations confirmed that these were gain-of-function mutations. Further studies in cohorts with low LDL cholesterol (LDLc) levels from large epidemiological cardiovascular studies reported that loss-of-function mutations in PCSK9 were associated with protection from cardiovascular disease. An additional critical observation provided evidence that the interaction of PCSK9 and the LDL receptor was through circulating, not intracellular, PCSK9, which bound to the receptor, and then mediated the recycling of the LDL receptor. These findings established PSCK9 as a potential therapeutic target and resulted in biopharmaceutical companies developing interventions designed to lower LDLc levels. Clinical development programs for monoclonal antibodies against PCSK9 have advanced rapidly with completion of comprehensive phase 1 and 2 trials with both REGN727/SAR236553 (REGN727) and AMG 145, clearly demonstrating substantial reductions in LDLc levels in patients receiving diet alone, low, moderate, and high doses of statins, or statin combined with ezetimibe, and both heterozygous familial hypercholesterolemia and nonfamilial hypercholesterolemia subjects. Concomitant and parallel reductions in the levels of apolipoprotein B and its related lipoproteins, and small but significant increases in HDL cholesterol levels were seen as anticipated. An unanticipated and robust decrease in lipoprotein(a) levels was also noted. Although these trials have been relatively short term, no significant safety issues or target organs of interest have emerged. Larger and much longer phase 3 trials are now in progress to assess the long-term tolerability, safety, and impact on cardiovascular disease events of these very effective LDLc lowering compounds.


Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of outstanding importance
Abifadel M, Varret M, Rabès JP, Allard D, Ouguerram K, Devillers M, et al. Mutations in PCSK9 cause autosomal dominant hypercholesterolemia. Nat Genet. 2003;34:154–6.
Maxwell KN, Breslow JL. Adenoviralmediated expression of Pcsk9 in mice results in a low-density lipoprotein receptor knockout phenotype. Proc Natl Acad Sci USA. 2004;101:7100–5.
Rashid S, Curtis DE, Garuti R, Anderson NN, Bashmakov Y, Ho YK, et al. Decreased plasma cholesterol and hypersensitivity to statins in mice lacking Pcsk9. Proc Natl Acad Sci USA. 2005;102:5374–9.
Cohen J, Pertsemlidis A, Kotowski IK, Graham R, Garcia CK, Hobbs HH. Low LDL cholesterol in individuals of African descent resulting from frequent nonsense mutations in PCSK9. Nat Genet. 2005;37:161–5.
Cohen JC, Boerwinkle E, Mosley Jr TH, Hobbs HH. Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. N Engl J Med. 2006;354:1264–72.
Zhao Z, Tuakli-Wosornu Y, Lagace TA, Kinch L, Grishin NV, Horton JD, et al. Molecular characterization of loss-of-function mutations in PCSK9 and identification of a compound heterozygote. Am J Hum Genet. 2006;79:514–23.
Hooper AJ, Marais AD, Tanyanyiwa DM, Burnett JR. The C679X mutation in PCSK9 is present and lowers blood cholesterol in a southern African population. Atherosclerosis. 2007;193:445–8.
Lagace TA, Curtis DE, Garuti R, McNutt MC, Park SW, Prather HB, et al. Secreted PCSK9 decreases the number of LDL receptors in hepatocytes and in livers of parabiotic mice. J Clin Invest. 2006;116:2995–3005.
Lambert G, Sjouke B, Choque B, Kastelein JJ, Hovingh GK. The PCSK9 decade: thematic review series: new lipid and lipoprotein targets for the treatment of cardiometabolic diseases. J Lipid Res. 2012;53(12):2515–24.
Seidah NG, Benjannet S, Wickham L, Marcinkiewicz J, Bélanger Jasmin S, Stifani S, et al. The secretory proprotein convertase neural apoptosis-regulated convertase 1 (NARC-1): liver regeneration and neuronal differentiation. Proc Natl Acad Sci USA. 2003;100:928–33.
Tremblay AJ, Lamarche B, Lemelin V, Hoos L, Benjannet S, Seidah NG, et al. Atorvastatin increases intestinal expression of NPC1L1 in hyperlipidemic men. J Lipid Res. 2011;52:558–65.
Careskey HE, Davis RA, Alborn WE, Troutt JS, Cao G, Konrad RJ. Atorvastatin increases human serum levels of proprotein convertase subtilisin/kexin type 9. J Lipid Res. 2008;49:394–8.
Li H, Li H, Ziegler N, Cui R, Liu J. Recent patents on PCSK9: a new target for treating hypercholesterolemia. Recent Pat DNA Gene Seq. 2009;3:201–12.
Benjannet S, Rhainds D, Essalmani R, Mayne J, Wickham L, Jin W, et al. NARC-1/PCSK9 and its natural mutants: zymogen cleavage and effects on the low density lipoprotein (LDL) receptor and LDL cholesterol. J Biol Chem. 2004;279:48865–75.
Qian YW, Schmidt RJ, Zhang Y, Chu S, Lin A, Wang H, et al. Secreted PCSK9 downregulates low density lipoprotein receptor through receptor-mediated endocytosis. J Lipid Res. 2007;48:1488–98.
Zhang DW, Lagace TA, Garuti R, Zhao Z, McDonald M, Horton JD, et al. Binding of proprotein convertase subtilisin/kexin type 9 to epidermal growth factor-like repeat A of low density lipoprotein receptor decreases receptor recycling and increases degradation. J Biol Chem. 2007;282:18602–12.
Kwon HJ, Lagace TA, McNutt MC, Horton JD, Deisenhofer J. Molecular basis for LDL receptor recognition by PCSK9. Proc Natl Acad Sci USA. 2008;105:1820–5.
Chen Y, Wang H, Yu L, Yu X, Qian YW, Cao G, et al. Role of ubiquitination in PCSK9-mediated low-density lipoprotein receptor degradation. Biochem Biophys Res Commun. 2011;415:515–8.
Lo Surdo P, Bottomley MJ, Calzetta A, Settembre EC, Cirillo A, Pandit S, et al. Mechanistic implications for LDL receptor degradation from the PCSK9/LDLR structure at neutral pH. EMBO Rep. 2011;12:1300–5.
• Tveten K, Holla OL, Cameron J, Strøm TB, Berge KE, Laerdahl JK, et al. Interaction between the ligand-binding domain of the LDL receptor and the C-terminal domain of PCSK9 is required for PCSK9 to remain bound to the LDL receptor during endosomal acidification. Hum Mol Genet. 2012;21:1402–9. This article provides potential information to make possible disruption of the PCSK9/LDL receptor interaction for therapeutic intervention.
Wang Y, Huang Y, Hobbs HH, Cohen JC. Molecular characterization of proprotein convertase subtilisin/kexin type 9-mediated degradation of the LDLR. J Lipid Res. 2012;53:1932–43.
Essalmani R, Susan-Resiga D, Chamberland A, Abifadel M, Creemers JW, Boileau C, et al. In vivo evidence that furin from hepatocytes inactivates PCSK9. J Biol Chem. 2011;286:4257–63.
Cameron J, Bogsrud MP, Tveten K, Strøm TB, Holven K, Berge KE, et al. Serum levels of proprotein convertase subtilisin/kexin type 9 in subjects with familial hypercholesterolemia indicate that proprotein convertase subtilisin/kexin type 9 is cleared from plasma by low-density lipoprotein receptor-independent pathways. Transl Res. 2012;160:125–30.
•• Stein EA, Mellis S, Yancopoulos GD, Stahl N, Logan D, Smith WB, et al. Effect of a monoclonal antibody to PCSK9 on LDL cholesterol. N Engl J Med. 2012;366:1108–18. This article reports the first studies in humans of therapy targeted at preventing PCSK9 binding to the LDL receptor and provides a proof of concept of efficacy to lower LDLc levels in patients receiving diet only, statin-stable patients, and patients with HeFH.
• Dias CS, Shaywitz AJ, Wasserman SM, Smith BP, Gao B, Stolman DS, et al. Effects of AMG 145 on low-density lipoprotein cholesterol levels: results from 2 randomized, double-blind, placebo-controlled, ascending-dose phase 1 studies in healthy volunteers and hypercholesterolemic subjects on statins. J Am Coll Cardiol. 2012;60:1888–98. A second human monoclonal antibody demonstrating efficacy in phase 1 and illustrating the relationship between higher doses and duration of effect.
• McKenney JM, Koren MJ, Kereiakes DJ, Hanotin C, Ferrand AC, Stein EA. Safety and efficacy of a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 serine protease, SAR236553/REGN727, in patients with primary hypercholesterolemia receiving ongoing stable atorvastatin therapy. J Am Coll Cardiol. 2012;59:2344–53. First published phase 2 data using a human monoclonal antibody -REGN727- in atorvastatin-treated hypercholesterolemic patients showing a robust reduction in LDLc levels.
• Stein EA, Gipe D, Bergeron J, Gaudet D, Weiss R, Dufour R, et al. Effect of a monoclonal antibody to PCSK9, REGN727/SAR236553, to reduce low-density lipoprotein cholesterol in patients with heterozygous familial hypercholesterolaemia on stable statin dose with or without ezetimibe therapy: a phase 2 randomised controlled trial. Lancet. 2012;380:29–36. This is the first specific study showing HeFH patients already receiving maximal lipid-lowering therapy achieve substantial additional reductions in LDLc levels.
•• Roth EM, McKenney JM, Hanotin C, Asset G, Stein EA. Atorvastatin with or without an antibody to PCSK9 in primary hypercholesterolemia. N Engl J Med. 2012;367:1891–900. doi:10.1056/NEJMoa1201832. This is a comparison of treating patients with an LDLc level of 100 mg/dL or greater after treatment with 10 mg atorvastatin for at least 7 weeks with 80 mg atorvastatin alone, 80 mg atorvastatin plus monoclonal antibodies, REGN727, or 10 mg atorvastatin plus REGN727. It clearly demonstrates the much greater reduction in LDLc levels obtained with REGN727 compared with an eightfold increase in statin dose.
Raal F, Scott R, Somaratne R, Bridges I, Li G, Wasserman SM, et al. Low-density lipoprotein cholesterol–lowering effects of AMG 145, a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 serine protease in patients with heterozygous familial hypercholesterolemia: the reduction of LDL-C with PCSK9 inhibition in heterozygous familial hypercholesterolemia disorder (RUTHERFORD) randomized trial. Circulation. 2012;126:2408–17.
•• Sullivan D, Olsson AG, Scott R, Kim JB, Xue A, Gebski V, et al. Effect of AMG 145, a monoclonal antibody to PCSK9, on LDL cholesterol levels in statin-intolerant patients: the GAUSS randomized trial. JAMA. 2012;308(23):2497–506. doi:10.1001/jama.2012.25790. This provides clear evidence of the first potential option for effective LDLc level reduction in patients unable to tolerate statins, or effective doses of statins.
Koren MJ, Scott R, Kim JB, Knusel B, Liu T, Lei L, et al. Efficacy, safety, and tolerability of a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 as monotherapy in patients with hypercholesterolaemia(MENDEL): a randomised, double-blind, placebo-controlled, phase 2 study. Lancet. 2012;380:1995–2006. doi:10.1016/S0140-6736(12)61771-1.
• Giugliano RP, Desai NR, Kohli P, Rogers WJ, Somaratne R, Huang F, et al. for the LAPLACE-TIMI 57 Investigators. Efficacy, safety, and tolerability of a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 in combination with a statin in patients with hypercholesterolaemia (LAPLACE-TIMI 57): a randomised, placebo-controlled, dose-ranging, phase 2 study. Lancet. 2012;380:2007–17. doi:10.1016/S0140-6736(12)61770-X. This is the largest and most global phase 2 trial done with the monoclonal antibody AMG 145, confirming LDLc efficacy and good tolerability compared with placebo.
Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502.
Dubuc G, Chamberland A, Wassef H, Davignon J, Seidah NG, Bernier L, et al. Statins upregulate PCSK9, the gene encoding the proprotein convertase neural apoptosis-regulated convertase-1 implicated in familial hypercholesterolemia. Arterioscler Thromb Vasc Biol. 2004;24:1454–9.
World Health Organization. Familial hypercholesterolemia (FH). Report of a second WHO communication 1999. WHO/HGN/FH/CONS99.2. Geneva: World Health Organization.
Roberts WC. The rule of 5 and the rule of 7 in lipid-lowering by statin drugs. Am J Cardiol. 1997;80:106–7.
Scharnagl H, Nauck M, Wieland H, Marz W. The Friedewald formula underestimates LDL cholesterol at low concentrations. Clin Chem Lab Med. 2001;39:426–31.
Turner T, Plunkett N, Miller J, Fearn J, Stein EA. Validity of calculated LDL cholesterol by the Friedewald formula compared to LDL cholesterol measured by ultracentrifugation in patients with very low LDL. Abstract A-105. http://www.aacc.org/events/annualmtgdirectory/Documents/AACC_12_Abstracts_A77-A119.pdf. Accessed 19 Oct 2012.
National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–421.
Firth JC, Marais AD. Familial hypercholesterolaemia: the Cape Town experience. S Afr Med J. 2008;98:99–104.
Scientific Steering Committee on behalf of the Simon Broome Register Group. Risk of fatal coronary heart disease in familial hypercholesterolaemia. BMJ. 1991;303:893–6.
Stein EA, Strutt K, Southworth H, Diggle PJ, Miller E, for the HeFH Study Group. Comparison of rosuvastatin versus atorvastatin in patients with heterozygous familial hypercholesterolemia. Am J Cardiol. 2003;92:1287–93.
Marais DA, Raal FJ, Stein EA, Rader DJ, Blasetto J, Palmer M, et al. A dose titration and comparative study of rosuvastatin and atorvastatin in patients with homozygous familial hypercholesterolemia. Atherosclerosis. 2007;197:400–6.
Regeneron Pharmaceuticals, Inc. Sanofi and Regeneron launch comprehensive phase 3 clinical program with LDL cholesterol-lowering PCSK9 antibody. http://investor.regeneron.com/releasedetail.cfm?ReleaseID=693585 (2012). Accessed 15 Nov 2012.
Acknowledgment
Prime Medica Ltd (Knutsford, Cheshire, UK) funded by Sanofi and Regeneron who provided limited editorial support.
Disclosure
E.A. Stein: has received consulting fees from Amgen, Adnexus Therapeutics, BMS, Genentech, and Regeneron/Sanofi relating to PCSK9 inhibitors and his institution has received research funding related to PCSK9 clinical trials from Alnylam, Amgen, BMS, Genentech, Sanofi, and Regeneron; G.D. Swergold: is an employee of Regeneron Pharmaceuticals, Inc.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Nonstatin Drugs
Rights and permissions
About this article
Cite this article
Stein, E.A., Swergold, G.D. Potential of Proprotein Convertase Subtilisin/Kexin Type 9 Based Therapeutics. Curr Atheroscler Rep 15, 310 (2013). https://doi.org/10.1007/s11883-013-0310-3
Published:
DOI: https://doi.org/10.1007/s11883-013-0310-3