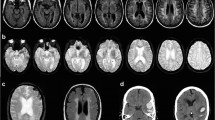
Abstract
Cerebral amyloid angiopathy (CAA) is defined as the deposition of amyloid ß peptide within leptomeningial and cortical vessels, likely reflecting an imbalance between Aβ production and clearance. Amyloid buildup triggers a series of destructive alterations in the cerebral vascular architecture, leading to a spectrum of neurological events including lobar intracerebral hemorrhage, brain ischemia and cognitive decline. Although traditionally diagnosed pathologically, neuroimaging has taken a central role in defining CAA. This review will discuss the pathological, clinical and radiological aspects of CAA.


Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Vinters HV. Cerebral amyloid angiopathy. A critical review. Stroke. 1987;18:311–24.
Okazaki H, Reagan TJ, Campbell RJ. Clinicopathologic studies of primary cerebral amyloid angiopathy. Mayo Clin Proc. 1979;54(1):22–31.
Pfeifer LA, White LR, Ross GW, et al. Cerebral amyloid angiopathy and cognitive function: the HAAS autopsy study. Neurology. 2002;58(11):1629–34.
Neuropathology Group. Medical Research Council Cognitive Function and Aging Study. Pathological correlates of late-onset dementia in a multicentre, community-based population in England and Wales. Neuropathology Group of the Medical Research Council Cognitive Function and Ageing Study (MRC CFAS). Lancet. 2001;357(9251):169–75.
• Kimberly WT, Gilson A, Rost NS, et al. Silent ischemic infarcts are associated with hemorrhage burden in cerebral amyloid angiopathy. Neurology. 2009;72(14):1230–5. This recent study demonstrated that ICH, especially CAA related, predisposes to clinically silent cerebral microinfarction as well as intracerebral hemorrhage, suggesting that the lifetime burden of ischemic infarction in CAA could be substantial and might contribute to small vessel-related cognitive impairment.
• Gregoire SM, Charidimou A, Gadapa N, et al. Acute ischaemic brain lesions in intracerebral haemorrhage: multicentre cross-sectional magnetic resonance imaging study. Brain. 2011;134:2376–86. This recent study demonstrated that ICH, especially CAA related, predisposes to clinically silent cerebral microinfarction as well as intracerebral hemorrhage, suggesting that the lifetime burden of ischemic infarction in CAA could be substantial and might contribute to small vessel-related cognitive impairment.
• Prabhakaran S, Gupta R, Ouyang B, et al. Acute brain infarcts after spontaneous intracerebral hemorrhage: a diffusion-weighted imaging study. Stroke. 2010;41(1):89–94. This recent study demonstrated that ICH, especially CAA related, predisposes to clinically silent cerebral microinfarction as well as intracerebral hemorrhage, suggesting that the lifetime burden of ischemic infarction in CAA could be substantial and might contribute to small vessel-related cognitive impairment.
Jellinger KA. Alzheimer disease and cerebrovascular pathology: an update. J Neural Transm. 2002;109(5–6):813–36.
Herzig MC, Van Nostrand WE, Jucker M. Mechanism of cerebral beta-amyloid angiopathy: murine and cellular models. Brain Pathol. 2006;16(1):40–54.
Fukuchi K, Ho L, Younkin SG, et al. High levels of circulating beta-amyloid peptide do not cause cerebral beta-amyloidosis in transgenic mice. Am J Pathol. 1996;149(1):219–27.
Burgermeister P, Calhoun ME, Winkler DT, Jucker M. Mechanisms of cerebrovascular amyloid deposition. Lessons from mouse models. Ann N Y Acad Sci. 2000;903:307–16.
Eisele YS, Obermüller U, Heilbronner G, et al. Peripherally applied Abeta-containing inoculates induce cerebral beta-amyloidosis. Science. 2010;330(6006):980–2.
Garcia-Alloza M, Gregory J, Kuchibhotla KV, et al. Cerebrovascular lesions induce transient β-amyloid deposition. Brain. 2011;134:3697–707.
Price DL, Tanzi RE, Borchelt DR, Sisodia SS. Alzheimer’s disease: genetic studies and transgenic models. Annu Rev Genet. 1998;32:461–93.
Greenberg SM, Nandigam RN, Delgado P, et al. Microbleeds versus macrobleeds: evidence for distinct entities. Stroke. 2009;40(7):2382–6.
Ishii K, Tamaoka A, Mizusawa H, et al. Abeta1-40 but not Abeta1-42 levels in cortex correlate with apolipoprotein E epsilon4 allele dosage in sporadic Alzheimer’s disease. Brain Res. 1997;748:250–2.
Love S, Miners S, Palmer J, et al. Insights into thepathogenesis and pathogenicity of cerebral amyloid angiopathy. Front Biosci. 2009;14:4778–92.
Masuda J, Tanaka K, Ueda K, Omae T. Autopsy study of incidence and distribution of cerebral amyloid angiopathy in Hisayama, Japan. Stroke. 1988;19(2):205–10.
Vinters HV, Gilbert JJ. Cerebral amyloid angiopathy: incidence and complications in the aging brain. II. The distribution of amyloid vascular changes. Stroke. 1983;14(6):924–8.
Van Broeck B, Van Broeckhoven C, Kumar-Singh S. Current insights into molecular mechanisms of Alzheimer disease and their implications for therapeutic approaches. Neurodegener Dis. 2007;4(5):349–65.
Mandybur TI. Cerebral amyloid angiopathy: the vascular pathology and complications. J Neuropathol Exp Neurol. 1986;45(1):79–90.
Vonsattel JP, Myers RH, Hedley-Whyte ET, et al. Cerebral amyloid angiopathy without and with cerebral hemorrhages: a comparative histological study. Ann Neurol. 1991;30:637–49.
Revesz T, Ghiso J, Lashley T, et al. Cerebral amyloid angiopathies: a pathologic, biochemical, and genetic view. J Neuropathol Exp Neurol. 2003;62:885–98.
Hartz AM, Bauer B, Soldner EL, et al. Amyloid-β contributes to blood–brain barrier leakage in transgenic human amyloid precursor protein mice and in humans with cerebral amyloid angiopathy. Stroke. 2012;43:514–23.
Puchtler H, Waldrop FS, Meloan SN. A review of light, polarization and fluorescence microscopic methods for amyloid. Appl Pathol. 1985;3:5–17.
Maia LF, Mackenzie IR, Feldman HH. Clinical phenotypes of cerebral amyloid angiopathy. J Neurol Sci. 2007;257:23–30.
Qureshi AI, Tuhrim S, Broderick JP, et al. Spontaneous intracerebral hemorrhage. N Engl J Med. 2001;344:1450–60.
Vinters HV, Wang ZZ, Secor DL. Brain parenchymal and microvascular amyloid in Alzheimer’s disease. Brain Pathol. 1996;6:179–95.
Winkler DT, Bondolfi L, Herzig MC, et al. Spontaneous hemorrhagic stroke in a mouse model of cerebral amyloid angiopathy. J Neurosci. 2001;21:1619–27.
Greenberg SM, Vonsattel JP, Stakes JW, et al. The clinical spectrum of cerebral amyloid angiopathy: presentations without lobar hemorrhage. Neurology. 1993;43:2073–9.
Yamada M, Itoh Y, Otomo E, et al. Subarachnoid haemorrhage in the elderly: a necropsy study of the association with cerebral amyloid angiopathy. J Neurol Neurosurg Psychiatry. 1993;56:543–7.
Linn J, Herms J, Dichgans M, et al. Subarachnoid hemosiderosis and superficial cortical hemosiderosis in cerebral amyloid angiopathy. AJNR Am J Neuroradiol. 2008;29:184–6.
Finelli PF. Cerebral amyloid angiopathy as cause of convexity SAH in elderly. Neurologist. 2010;16:37–40.
Linn J, Halpin A, Demaerel P, et al. Prevalence of superficial siderosis in patients with cerebral amyloid angiopathy. Neurology. 2010;74:1346–50.
Raposo N, Viguier A, Cuvinciuc V, et al. Cortical subarachnoid haemorrhage in the elderly: a recurrent event probably related to cerebral amyloid angiopathy. Eur J Neurol. 2011;18:597–603.
Greenberg SM, Rebeck GW, Vonsattel JP, et al. Apolipoprotein E epsilon 4 and cerebral hemorrhage associated with amyloid angiopathy. Ann Neurol. 1995;38:254–9.
Premkumar DR, Cohen DL, Hedera P, et al. Apolipoprotein E-epsilon4 alleles in cerebral amyloid angiopathy and cerebrovascular pathology associated with Alzheimer’s disease. Am J Pathol. 1996;148:2083–95.
McCarron MO, Nicoll JA, Ironside JW, et al. Cerebral amyloid angiopathy-related hemorrhage. Interaction of APOE epsilon2 with putative clinical risk factors. Stroke. 1999;30:1643–6.
Nicoll JA, Burnett C, Love S, et al. High frequency of apolipoprotein E epsilon 2 allele in hemorrhage due to cerebral amyloid angiopathy. Ann Neurol. 1997;41:716–21.
McCarron MO, Nicoll JA, Ironside JW, et al. Cerebral amyloid angiopathy-related hemorrhage. Interaction of APOE epsilon2 with putative clinical risk factors. Stroke. 1999;30:1643–6.
Holtzman DM. Role of apoe/Abeta interactions in the pathogenesis of Alzheimer’s disease and cerebral amyloid angiopathy. J Mol Neurosci. 2001;17:147–55.
Rosand J, Hylek EM, O’Donnell HC, Greenberg SM. Warfarin-associated hemorrhage and cerebral amyloid angiopathy: a genetic and pathologic study. Neurology. 2000;55:947–51.
Eckman MH, Wong LK, Soo YO, et al. Patient-specific decision-making for warfarin therapy in nonvalvular atrial fibrillation: how will screening with genetics and imaging help? Stroke. 2008;39:3308–15.
Biffi A, Shulman JM, Jagiella JM, et al. Genetic variation at CR1 increases risk of cerebral amyloid angiopathy. Neurology. 2012;78:334–41.
• Arima H, Tzourio C, Anderson C, PROGRESS Collaborative Group, et al. Effects of perindopril-based lowering of blood pressure on intracerebral hemorrhage related to amyloid angiopathy: the PROGRESS trial. Stroke. 2010;41:394–6. Currently there is no preventive strategy for CAA related ICH. In this subsidiary analysis of the PROGRESS trial routine blood pressure reduction was shown to protect patients with CAA from ICH.
Salloway S, Sperling R, Gilman S, et al. Bapineuzumab 201 Clinical Trial Investigators. A phase 2 multiple ascending dose trial of bapineuzumab in mild to moderate. Alzheimer disease. Neurology. 2009;73:2061–70.
Bayer AJ, Bullock R, Jones RW, et al. Evaluation of the safety and immunogenicity of synthetic Abeta42 (AN1792) in patients with AD. Neurology. 2005;64:94–101.
Biffi A, Greenberg SM. Cerebral amyloid angiopathy: a systematic review. J Clin Neurol. 2011;7:1–9.
Eckman MH, Rosand J, Knudsen KA, et al. Can patients be anticoagulated after intracerebral hemorrhage? A decision analysis. Stroke. 2003;34:1710–6.
Biffi A, Halpin A, Towfighi A, et al. Aspirin and recurrent intracerebral hemorrhage in cerebral amyloid angiopathy. Neurology. 2010;75:693–8.
Fisher M, Vasilevko V, Passos GF, et al. Therapeutic modulation of cerebral microhemorrhage in a mouse model of cerebral amyloid angiopathy. Stroke. 2011;42:3300–3.
Knudsen KA, Rosand J, Karluk D, Greenberg SM. Clinical diagnosis of cerebral amyloid angiopathy: validation of the Boston criteria. Neurology. 2001;56:537–9.
Greenberg SM, Vernooij MW, Cordonnier C, Microbleed Study Group, et al. Cerebral microbleeds: a guide to detection and interpretation. Lancet Neurol. 2009;8(2):165–74.
Greenberg SM, Eng JA, Ning M, et al. Hemorrhage burden predicts recurrent intracerebral hemorrhage after lobar hemorrhage. Stroke. 2004;35:1415–20.
Rosand J, Muzikansky A, Kumar A, et al. Spatial clustering of hemorrhages in probable cerebral amyloid angiopathy. Ann Neurol. 2005;58:459–62.
Mesker DJ, Poels MM, Ikram MA, et al. Lobar distribution of cerebral microbleeds: the Rotterdam Scan Study. Arch Neurol. 2011;68:656–9.
Smith EE, Greenberg SM. Clinical diagnosis of cerebral amyloid angiopathy: validation of the Boston criteria. Curr Atheroscler Rep. 2003;5:260–6.
Vernooij MW, Ikram MA, Hofman A, et al. Superficial siderosis in the general population. Neurology. 2009;73:202–5.
Klohs J, Deistung A, Schweser F, et al. Detection of cerebral microbleeds with quantitative susceptibility mapping in the ArcAbeta mouse model of cerebral amyloidosis. J Cereb Blood Flow Metab. 2011;31:2282–92.
Schrag M, McAuley G, Pomakian J, et al. Correlation of hypointensities in susceptibility-weighted images to tissue histology in dementia patients with cerebral amyloid angiopathy: a postmortem MRI study. Acta Neuropathol. 2010;119:291–302.
Johnson KA, Gregas M, Becker JA, et al. Imaging of amyloid burden and distribution in cerebral amyloidangiopathy. Ann Neurol. 2007;62:229–34.
Ly JV, Donnan GA, Villemagne VL, et al. 11C-PIB binding is increased in patients with cerebral amyloid angiopathy-related hemorrhage. Neurology. 2010;74(6):487–93.
Smith EE, Vijayappa M, Lima F, et al. Impaired visual evoked flow velocity response in cerebral amyloid angiopathy. Neurology. 2008;71:1424–30.
Thomas T, Thomas G, McLendon C, et al. beta-Amyloid-mediated vasoactivity and vascular endothelial damage. Nature. 1996;380:168–71.
Christie R, Yamada M, Moskowitz M, Hyman B. Structural and functional disruption of vascular smooth muscle cells in a transgenic mouse model of amyloid angiopathy. Am J Pathol. 2001;158:1065–71.
Smith EE, Gurol ME, Eng JA, et al. White matter lesions, cognition, and recurrent hemorrhage in lobar intracerebral hemorrhage. Neurology. 2004;63:1606–12.
Pantoni L. Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol. 2010;9:689–701.
Natté R, Maat-Schieman ML, Haan J, et al. Dementia in hereditary cerebral hemorrhage with amyloidosis-Dutch type is associated with cerebral amyloid angiopathy but is independent of plaques and neurofibrillary tangles. Ann Neurol. 2001;50:765–72.
Gorelick PB, Scuteri A, Black SE, American Heart Association Stroke Council, Council on Epidemiology and Prevention, Council on Cardiovascular Nursing, et al. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42:2672–713.
Werring DJ, Gregoire SM, Cipolotti L. Cerebral microbleeds and vascular cognitive impairment. J Neurol Sci. 2010;299:131–5.
Holland CM, Smith EE, Csapo I, et al. Spatial distribution of white-matter hyperintensities in Alzheimer disease, cerebral amyloid angiopathy, and healthy aging. Stroke. 2008;39:1127–33.
Soontornniyomkij V, Lynch MD, Mermash S, et al. Cerebral microinfarcts associated with severe cerebral beta-amyloid angiopathy. Brain Pathol. 2010;20:459–67.
Viswanathan A, Patel P, Rahman R, et al. Tissue microstructural changes are independently associated with cognitive impairment in cerebral amyloid angiopathy. Stroke. 2008;39:1988–92.
• Arvanitakis Z, Leurgans SE, Wang Z, et al. Cerebral amyloid angiopathy pathology and cognitive domains in older persons. Ann Neurol. 2011;69:320–7. This is an up to date clinical-pathologic study of more than 400 older persons showing that CAA is very common in the demented and the non-demented population and is associated with AD, and after controlling for AD, with decline in specific cognitive performances.
Palsdottir A, Snorradottir AO, Thorsteinsson L. Hereditary cystatin C amyloid angiopathy: genetic, clinical, and pathological aspects. Brain Pathol. 2006;16:55–9.
Roks G, Van Harskamp F, De Koning I, et al. Presentation of amyloidosis in carriers of the codon 692 mutation in the amyloid precursor protein gene (APP692). Brain. 2000;123:2130–40.
Vidal R, Frangione B, Rostagno A, et al. A stop-codon mutation in the BRI gene associated with familial British dementia. Nature. 1999;399:776–81.
Vidal R, Garzuly F, Budka H, et al. Meningocerebrovascular amyloidosis associated with a novel transthyretin mis-sense mutation at codon 18 (TTRD 18G). Am J Pathol. 1996;148:361–6.
Zhang-Nunes SX, Maat-Schieman ML, van Duinen SG, et al. The cerebral beta-amyloid angiopathies: hereditary and sporadic. Brain Pathol. 2006;16:30–9.
Greenberg SM, Shin Y, Grabowski TJ, et al. Hemorrhagic stroke associated with the Iowa amyloid precursor protein mutation. Neurology. 2003;60:1020–2.
Fountain NB, Eberhard DA. Primary angiitis of the central nervous system associated with cerebral amyloid angiopathy: report of two cases and review of the literature. Neurology. 1996;46:190–7.
Eng JA, Frosch MP, Choi K, et al. Clinical manifestations of cerebral amyloid angiopathy-related inflammation. Ann Neurol. 2004;55:250–6.
Kinnecom C, Lev MH, Wendell L, et al. Course of cerebral amyloid angiopathy-related inflammation. Neurology. 2007;68:1411–6.
Kloppenborg RP, Richard E, Sprengers ME, et al. Steroid responsive encephalopathy in cerebral amyloid angiopathy: a case report and review of evidence for immunosuppressive treatment. J Neuroinflammation. 2010;7:18.
• DiFrancesco JC, Brioschi M, Brighina L, et al. Anti-Aβ autoantibodies in the CSF of a patient with CAA-related inflammation: a case report. Neurology. 2011;76:842–4. This case report demonstrated for the first time that CSF anti-Aβ antibodies might serve as a biological marker for the diagnosis, monitoring and evaluation of treatment in CAA-related inflammation.
Greenberg SM, Frosch MP. Life imitates art: anti-amyloid antibodies and inflammatory cerebral amyloid angiopathy. Neurology. 2011;76:772–3.
Disclosure
No potential conflicts of interest related to this article were reported.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
Boston Criteria for Diagnosis of CAA-Related Hemorrhage [52]
-
1.
Definite CAA
Full postmortem examination demonstrating:
Lobar, cortical, or corticosubcortical hemorrhage
Severe CAA with vasculopathy
Absence of other diagnostic lesion
-
2.
Probable CAA with supporting pathology
Clinical data and pathologic tissue (evacuated hematoma or cortical biopsy) demonstrating:
Lobar, cortical, or corticosubcortical hemorrhage
Some degree of CAA in specimen
Absence of other diagnostic lesion
-
3.
Probable CAA
Clinical data and MRI or CT demonstrating:
Multiple hemorrhages restricted to lobar, cortical, or corticosubcortical regions (cerebellar hemorrhage allowed)
Age ≥55 years
Absence of other cause of hemorrhage
-
4.
Possible CAA
Clinical data and MRI or CT demonstrating:
Single lobar, cortical, or corticosubcortical hemorrhage
Age ≥55 years
Absence of other cause of hemorrhage
Rights and permissions
About this article
Cite this article
Auriel, E., Greenberg, S.M. The Pathophysiology and Clinical Presentation of Cerebral Amyloid Angiopathy. Curr Atheroscler Rep 14, 343–350 (2012). https://doi.org/10.1007/s11883-012-0254-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11883-012-0254-z