Abstract
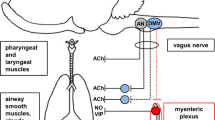
Neural regulation of the airways consists of cholinergic excitatory, adrenergic inhibitory nerves and nonadrenergic, noncholinergic (NANC) nerves. NANC nerves can be either inhibitory or excitatory. Cholinergic nerves form the predominant bronchoconstrictor neural pathway in human airways. Acetylcholine controls neuronal and nonneuronal target cells via a short-lived action at nicotinic and muscarinic receptors. The most important control over acetylcholine release from postganglionic cholinergic nerves is exerted by acetylcholine itself. The M2 autoreceptor is located prejunctionally on postganglionic nerves. Its stimulation limits the further release of acetylcholine. A loss of function in the neuronal muscarinic M2 autoreceptor occurs after exposure to allergen, ozone, or viruses. In human airways, inhibitory NANC (i-NANC) mechanisms are the only neural bronchodilatory mechanisms. The presumed neurotransmitters of the i-NANC system are vasoactive intestinal peptide and nitric oxide. Substance P and neurokinin A have been implicated as the neurotransmitters mediating the excitatory part of the NANC nervous system. NK2 receptors are present on smooth muscle of both large and small airways and mediate part of the bronchoconstrictor effect of tachykinins. Most of the proinflammatory effects of substance P are mediated by the NK1 receptor. Tachykinin receptor antagonists are currently being developed as a possible anti-asthma treatment. An extensive cross-talk exists between nerves and the immune system. The complexity of the picture has increased further as it has become clear that classical neurotransmitters, such as acetylcholine and neuropeptides, are produced by nonneuronal cells.
Similar content being viewed by others
References and Recommended Reading
Costello RW, Fryer AD: Cholinergic mechanisms in asthma. In Asthma. Edited by Barnes PJ, Grunstein MM, Leff AR, Woolcock AJ. Philadelphia: Lippincott Raven, 1997:965–984.
Klapproth H, Reinheimer T, Metzen J, et al.: Non-neuronal acetylcholine, a signalling molecule synthesised by surface cells of rat and man. Naunyn Schmiedebergs Arch Pharmacol 1997, 355:515–523.
Wessler I, Kirkpatrick CJ, Racke K: Non-neuronal acetylcholine, a locally acting molecule, widely distributed in biological systems: expression and function in humans. Pharmacol Ther 1998, 77:59–79. This is an extensive review of the experiments demonstrating that the cholinergic system is widely distributed in nonneuronal cells in humans. The synthesizing enzyme choline acetyltransferase, the signaling molecule acetylcholine, and the respective receptors (nicotinic or muscarinic) are expressed in epithelial cells, including those of the human airways.
Reinheimer T, Baumgartner D, Hohle KD, et al.: Acetylcholine via muscarinic receptors inhibits histamine release from human isolated bronchi. Am J Respir Crit Care Med 1997, 156:389–395.
D’Agostino G, Erbelding D, Kilbinger H: Tachykinin NK(2) receptors facilitate acetylcholine release from guinea-pig isolated trachea. Eur J Pharmacol 2000, 396:29–32.
Kakuyama M, Ahluwalia A, Rodrigo J, et al.: Cholinergic contraction is altered in nNOS knockouts: cooperative modulation of neural bronchoconstriction by nNOS and COX. Am J Respir Crit Care Med 1999, 160:2072–2078.
Song P, Milanese M, Crimi E, et al.: Allergen challenge of passively sensitized human bronchi alters M2 and beta2 receptor function. Am J Respir Crit Care Med 1997, 155:1230–1234.
Costello RW, Jacoby DB, Fryer AD: Pulmonary neuronal M2 muscarinic receptor function in asthma and animal models of hyperreactivity. Thorax 1998, 53:613–616.
Costello RW, Evans CM, Yost BL, et al.: Antigen-induced hyperreactivity to histamine: role of the vagus nerves and eosinophils. Am J Physiol 1999, 276:L709-L714.
Yost BL, Gleich GJ, Fryer AD: Ozone-induced hyperresponsiveness and blockade of M2 muscarinic receptors by eosinophil major basic protein. J Appl Physiol 1999, 87:1272–1278.
Jacoby DB, Xiao HQ, Lee NH, et al.: Virus- and interferoninduced loss of inhibitory M2 muscarinic receptor function and gene expression in cultured airway parasympathetic neurons. J Clin Invest 1998, 102:242–248.
Joos GF, Germonpre P, Pauwels RA: Neural mechanisms in asthma. Clin Exp Allergy 2000, 30(suppl 1):60–65.
O’Connor BJ, Towse LJ, Barnes PJ: Prolonged effect of tiotropium bromide on methacholine-induced bronchoconstriction in asthma. Am J Respir Crit Care Med 1996, 154:876–880.
Littner MR, Ilowite JS, Tashkin DP, et al.: Long-acting bronchodilation with once-daily dosing of tiotropium (Spiriva) in stable chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2000, 161:1136–1142.
van Noord JA, Bantje TA, Eland ME, et al.: A randomised controlled comparison of tiotropium and ipratropium in the treatment of chronic obstructive pulmonary disease: the Dutch Tiotropium Study Group. Thorax 2000, 55:289–294.
Belvisi MG: Bronchodilator nerves. In Asthma. Edited by Barnes PJ, Grunstein MM, Leff AR, Woolcock AJ. Philadelphia: Lippincott Raven; 1997:1027–1049.
Luts A, Uddman R, Alm P, et al.: Peptide-containing nerve fibers in human airways: distribution and coexistence pattern. Int Arch Allergy Immunol 1993, 101:52–60.
Kinhult J, Andersson JA, Uddman R, et al.: Pituitary adenylate cyclase-activating peptide 38 a potent endogenously produced dilator of human airways. Eur Respir J 2000, 15:243–247.
Pozo D, Delgado M, Martinez M, et al.: Immunobiology of vasoactive intestinal peptide (VIP). Immunol Today 2000, 21:7–11.
Kaltreider HB, Ichikawa S, Byrd PK, et al.: Upregulation of neuropeptides and neuropeptide receptors in a murine model of immune inflammation in lung parenchyma. Am J Respir Cell Mol Biol 1997, 16:133–144.
Chanez P, Springall DR, Vignola A, et al.: Bronchial mucosal immunoreactivity of sensory neuropeptides in severe airway diseases. Am J Respir Crit Care Med 1998, 158:985–990. This is an extensive study on the expression of peptidergic nerves, performed on endobronchial biopsy samples from a large group of normal volunteers, patients with asthma of varying severity, and patients with chronic bronchitis. This study could not confirm previous findings in autopsy material of some defects in sensory and VIP-containing nerves in severe asthma.
Lucchini RE, Facchini F, Turato G, et al.: Increased VIP-positive nerve fibers in the mucous glands of subjects with chronic bronchitis. Am J Respir Crit Care Med 1997, 156:1963–1968.
Fischer A, Hoffmann B: Nitric oxide synthase in neurons and nerve fibers of lower airways and in vagal sensory ganglia of man. Am J Respir Crit Care Med 1996, 154:209–216.
Michel T, Feron O: Nitric oxide synthases: which, where, how, and why? J Clin Invest 1997, 100:2146–2152.
Grasemann H, Yandava CN, Storm van’s GK, et al.: A neuronal NO synthase (NOS1) gene polymorphism is associated with asthma. Biochem Biophys Res Commun 2000, 272:391–394.
De Sanctis GT, Mehta S, Kobzik L, et al.: Contribution of type I NOS to expired gas NO and bronchial responsiveness in mice. Am J Physiol 1997, 273:L883-L888.
De Sanctis GT, MacLean JA, Hamada K, et al.: Contribution of nitric oxide synthases 1, 2, and 3 to airway hyperresponsiveness and inflammation in a murine model of asthma. J Exp Med 1999, 189:1621–1630.
Joos GF, Germonpre PR, Pauwels RA: Role of tachykinins in asthma. Allergy 2000, 55:321–337.
Sont JK, van Krieken JHJM, van Klink HCJ, et al.: Enhanced expression of neutral endopeptidase (NEP) in airway epithelium in biopsies from steroid-versus nonsteroidtreated patients with atopic asthma. Am J Respir Cell Mol Biol 1997, 16:549–556.
Krishna MT, Springall DR, Meng QH, et al.: Effects of ozone on epithelium and sensory nerves in the bronchial mucosa of healthy humans. Am J Respir Crit Care Med 1997, 156:943–950.
Baraniuk JN, Ali M, Yuta A, et al.: Hypertonic saline nasal provocation stimulates nociceptive nerves, substance P release, and glandular mucous exocytosis in normal humans. Am J Respir Crit Care Med 1999, 160:655–662.
Lilly CM, Bai TR, Shore SA, et al.: Neuropeptide content of lungs from asthmatic and nonasthmatic patients. Am J Respir Crit Care Med 1995, 151:548–553.
Maggi CA: The effects of tachykinins on inflammatory and immune cells. Regul Pept 1997, 70:75–90.
Joos GF, Pauwels RA: Pro-inflammatory effects of substance P: new perspectives for the treatment of airway diseases? Trends Pharmacol Sci 2000, 21:131–133.
Killingsworth CR, Shore SA, Alessandrini F, et al.: Rat alveolar macrophages express preprotachykinin gene-I mRNA-encoding tachykinins. Am J Physiol 1997, 273:L1073-L1081.
Ho WZ, Lai JP, Zhu XH, et al.: Human monocytes and macrophages express substance P and neurokinin-1 receptor. J Immunol 1997, 159:5654–5660.
Germonpre PR, Bullock GR, Lambrecht BN, et al.: Presence of substance P and neurokinin 1 receptors in human sputum macrophages and U-937 cells. Eur Respir J 1999, 14:776–782. This is the first demonstration of the presence of SP and the tachykinin NK1 receptor in human lung macrophages.
Lai JP, Douglas ST, Rappaport E, et al.: Identification of a delta isoform of preprotachykinin mRNA in human mononuclear phagocytes and lymphocytes. J Neuroimmunol 1998, 91:121–128.
Lai JP, Douglas SD, Ho WZ: Human lymphocytes express SP and its receptor. J Neuroimmunol 1998, 86:80–86.
de Giorgio R, Tazzari PL, Barbara G, et al.: Detection of substance P immunoreactivity in human peripheral leukocytes. J Neuroimmunol 1998, 82:175–181.
Lambrecht BN, Germonpre PR, Everaert EG, et al.: Endogenously produced substance P contributes to lymphocyte proliferation induced by dendritic cells and direct TCR ligation. Eur J Immunol 1999, 29:3815–3825. This article describes the production of SP by dendritic cells. Dendritic cells contain the mRNA and the peptide. Moreover, SP contributes to T-cell proliferation in a mixed leukocyte reaction.
Maggi CA: The troubled story of tachykinins and neurokinins. Trends Pharmacol Sci 2000, 21:173–175.
Advenier C, Joos G, Molimard M, et al.: Role of tachykinins as contractile agonists of human airways in asthma. Clin Exp Allergy 1999, 29:579–584.
Daoui S, Cognon C, Naline E, et al.: Involvement of tachykinin NK3 receptors in citric acid-induced cough and bronchial responses in guinea pigs. Am J Respir Crit Care Med 1998, 158:42–48.
Daoui S, Naline E, Lagente V, et al.: Neurokinin B- and specific tachykinin NK(3) receptor agonists-induced airway hyperresponsiveness in the guinea-pig. Br J Pharmacol 2000, 130:49–56.
Katsunuma T, Mak JCW, Barnes PJ: Glucocorticoids reduce tachykinin NK2 receptor expression in bovine tracheal smooth muscle. Eur J Pharmacol 1998, 344:99–106.
Katsunuma T, Roffel AF, Elzinga CR, et al.: Beta(2)-adrenoceptor agonist-induced upregulation of tachykinin NK(2) receptor expression and function in airway smooth muscle. Am J Respir Cell Mol Biol 1999, 21:409–417.
Mapp CE, Miotto D, Braccioni F, et al.: The distribution of neurokinin-1 and neurokinin-2 receptors in human central airways. Am J Respir Crit Care Med 2000, 161:207–215.
James DE, Nijkamp FP: Neuro-immune interactions in the lung. Clin Exp Allergy 1999, 29:1309–1319.
Suzuki R, Furuno T, McKay DM, et al.: Direct neurite-mast cell communication in vitro occurs via the neuropeptide substance P. J Immunol 1999, 163:2410–2415.
Cooke HJ, Fox P, Alferes L, et al.: Presence of NK1 receptors on a mucosal-like mast cell line, RBL-2H3 cells. Can J Physiol Pharmacol 1998, 76:188–193.
Forsythe P, McGarvey LP, Heaney LG, et al.: Sensory neuropeptides induce histamine release from bronchoalveolar lavage cells in both nonasthmatic coughers and cough variant asthmatics. Clin Exp Allergy 2000, 30:225–232.
Okada T, Hirayama Y, Kishi S, et al.: Functional neurokinin NK-1 receptor expression in rat peritoneal mast cells. Inflamm Res 1999, 48:274–279.
Marriott I, Mason MJ, Elhofy A, et al.: Substance P activates NF-kappaB independent of elevations in intracellular calcium in murine macrophages and dendritic cells. J Neuroimmunol 2000, 102:163–171.
Kradin R, MacLean J, Duckett S, et al.: Pulmonary response to inhaled antigen: neuroimmune interactions promote the recruitment of dendritic cells to the lung and the cellular immune response to inhaled antigen. Am J Pathol 1997, 150:1735–1743.
Schuiling M, Zuidhof A, Zaagsma J, et al.: Involvement of tachykinin NK1 receptor in the development of allergeninduced airway hyperreactivity and airway inflammation in conscious, unrestrained guinea-pigs. Am J Respir Crit Care Med 1999, 159:423–430.
Schuiling M, Zuidhof A, Meurs H, et al.: Role of tachykinin NK2-receptor activation in the allergen-induced late asthmatic reaction, airway hyperreactivity and airway inflammation cell influx in conscious, unrestrained guinea-pigs. Br J Pharmacol 1999, 127:1030–1038.
Maghni K, Taha R, Afif W, et al.: Dichotomy between neurokinin receptor actions in modulating allergic airway responses in an animal model of helper T cell type 2 cytokine-associated inflammation. Am J Respir Crit Care Med 2000, 162:1068–1074.
Ichinose M, Nakajima N, Takahashi T, et al.: Protection against bradykinin-induced bronchoconstriction in asthmatic patients by neurokinin receptor antagonist. Lancet 1992, 340:1248–1251.
Joos GF, Van Schoor J, Kips JC, et al.: The effect of inhaled FK244, a tachykinin NK-1 and NK-2 receptor antagonist, on neurokinin A-induced bronchoconstriction in asthmatics. Am J Respir Crit Care Med 1996, 153:1781–1784.
Fahy JV, Wong HH, Geppetti P, et al.: Effect of an NK1 receptor antagonist (CP-99,994) on hypertonic saline-induced bronchoconstriction and cough in male asthmatic subjects. Am J Respir Crit Care Med 1995, 152:879–884.
Ichinose M, Miura M, Yamauchi H, et al.: A neurokinin 1-receptor antagonist improves exercise-induced airway narrowing in asthmatic patients. Am J Respir Crit Care Med 1996, 153:936–941.
Van Schoor J, Joos G, Chasson B, et al.: The effect of the NK2 receptor antagonist SR48968 (saredutant) on neurokinin A-induced bronchoconstriction in asthmatics. Eur Respir J 1998, 12:17–23.
Blom HM, Severijnen LAWFM, Van Rijswijk JB, et al.: The long-term effects of capsaicin aqueous spray on the nasal mucosa. Clin Exp Allergy 1998, 28:1351–1358.
Caterina MJ, Schumacher MA, Tominaga M, et al.: The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 1997, 389:816–824.
Hwang SW, Cho H, Kwak J, et al.: Direct activation of capsaicin receptors by products of lipoxygenases: endogenous capsaicin-like substances. Proc Natl Acad Sci U S A 2000, 97:6155–6160.
Ellis JL, Sham JS, Undem BJ: Tachykinin-independent effects of capsaicin on smooth muscle in human isolated bronchi. Am J Respir Crit Care Med 1997, 155:751–755.
Biro T, Maurer M, Modarres S, et al.: Characterization of functional vanilloid receptors expressed by mast cells. Blood 1998, 91:1332–1340. In addition to its action on sensory neurons, capsaicin may also act directly on mast cells.
Bonini S, Lambiase A, Levi-Schaffer F, et al.: Nerve growth factor: an important molecule in allergic inflammation and tissue remodelling. Int Arch Allergy Immunol 1999, 118:159–162.
Braun A, Lommatzsch M, Renz H: The role of neurotrophins in allergic bronchial asthma. Clin Exp Allergy 2000, 30:178–186.
Virchow JC, Julius P, Lommatzsch M, et al.: Neurotrophins are increased in bronchoalveolar lavage fluid after segmental allergen provocation. Am J Respir Crit Care Med 1998, 158:2002–2005.
Sanico AM, Stanisz AM, Gleeson TD, et al.: Nerve growth factor expression and release in allergic inflammatory disease of the upper airways. Am J Respir Crit Care Med 2000, 161:1631–1635.
Braun A, Appel E, Baruch R, et al.: Role of nerve growth factor in a mouse model of allergic airway inflammation and asthma. Eur J Immunol 1998, 28:3240–3251. In this mouse model, allergic airway inflammation is accompanied by enhanced local production of NGF. NGF augmented TH2 effector functions. Nasal application of anti-NGF antibodies reduced IL-4 production and prevented development of tracheal hyperreactivity.
Hoyle GW, Graham RG, Finkelstein JB, et al.: Hyperinnervation of the airways in transgenic mice overexpressing nerve growth factor. Am J Respir Cell Mol Biol 1998, 18:149–157.
Hunter DD, Myers AC, Undem BJ: Nerve growth factor-induced phenotypic switch in guinea pig airway sensory neurons. Am J Respir Crit Care Med 2000, 161:1985–1990.
De Vries A, Dessing MC, Engels F, et al.: Nerve growth factor induces a neurokinin-1 receptor-mediated airway hyperresponsiveness in guinea pigs. Am J Respir Crit Care Med 1999, 159:1541–1544.
Knight DA, Lydell CP, Zhou D, et al.: Leukemia inhibitory factor (LIF) and LIF receptor in human lung: distribution and regulation of LIF release. Am J Respir Cell Mol Biol 1999, 20:834–841.
Knight DA, D’Aprile AC, Spalding LJ, et al.: Leukaemia inhibitory factor (LIF) upregulates excitatory non-adrenergic non-cholinergic and maintains cholinergic neural function in tracheal explants. Br J Pharmacol 2000, 130:975–979.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Joos, G.F. The role of neuroeffector mechanisms in the pathogenesis of asthma. Curr Allergy Asthma Rep 1, 134–143 (2001). https://doi.org/10.1007/s11882-001-0081-8
Issue Date:
DOI: https://doi.org/10.1007/s11882-001-0081-8