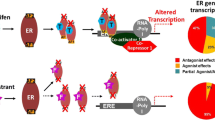
Opinion statement
It has become clear that patients whose cancers have progressed post-CDK4/6 inhibitor therapy (CDK4/6i) are not deriving the same magnitude of benefit to subsequent standard endocrine therapy as historical studies would suggest. For example, anticipated duration of benefit to fulvestrant prior to CDK4/6i historically was ~ 5–6 months, and data from the VERONICA and EMERALD trials report less than 2 months. This has magnified our need for novel endocrine agents. Some have argued that patients post-CDK4/6i may just have more endocrine-resistant tumors and perhaps should just receive chemotherapy. While this may be appropriate for some, we do not currently have an assay that reliably predicts whose cancers remain endocrine sensitive and whose are endocrine resistant. ESR1 mutations can enrich for patients whose tumors are more likely to be heavily dependent on estrogen, but this is certainly not the whole answer and many patients without ESR1 mutations continue to derive benefit from subsequent endocrine agents. Most patients would strongly prefer the side effect profile of endocrine agents compared to chemotherapy, and thus, premature use of cytotoxic agents when subsequent ER targeting can control disease is not preferred. These novel ER targeting agents (PROTAC, SERD, SERCA, CERAN) hold great promise to not only outperform standard agents like fulvestrant, but also offer the promise of agents with a different side effect profile that may be more advantageous when compared to menopausal symptoms, hot flashes, arthralgias, and sexual side effects so commonly seen with AIs. We also are likely to see these novel agents move to earlier lines, whether that be 1st line in combination with CDK4/6i or even adjuvant disease.

Similar content being viewed by others
Abbreviations
- AE:
-
Adverse event
- AI:
-
Aromatase inhibitor
- BC:
-
Breast cancer
- CBR:
-
Clinical benefit rate
- CDK4/6i:
-
Cyclin-dependent kinase 4/6 inhibitor
- CERAN:
-
Complete estrogen receptor antagonist
- ctDNA:
-
Circulating tumor DNA
- DLTs:
-
Dose-limiting toxicities
- EBC:
-
Early-stage breast cancer
- ER:
-
Estrogen receptor
- ET:
-
Endocrine therapy
- FIH:
-
First-in-human
- G:
-
Grade
- HR:
-
Hazard ratio
- HR + :
-
Hormone receptor-positive
- iDFS:
-
Invasive disease-free survival
- 1L:
-
1st line
- 2-3L:
-
2nd or 3rd line
- MBC:
-
Metastatic breast cancer
- ORR:
-
Overall response rate
- OS:
-
Overall survival
- PFS:
-
Progression-free survival
- PROTAC:
-
Proteolysis targeting chimera
- SERCA:
-
Selective estrogen receptor covalent antagonist
- SERD:
-
Selective estrogen receptor degrader
- SERM:
-
Selective estrogen receptor modulator
- WT:
-
Wild type
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Patel HK, Bihani T. Selective estrogen receptor modulators (SERMs) and selective estrogen receptor degraders (SERDs) in cancer treatment. Pharmacol Ther. 2018;186:1–24.
Heldring N, Pike A, Andersson S, et al. Estrogen receptors: how do they signal and what are their targets. Physiol Rev. 2007;87:905–31.
Hall JM, McDonnell DP. Coregulators in nuclear estrogen receptor action: from concept to therapeutic targeting. Mol Interv. 2005;5:343–57.
Carroll JS, Meyer CA, Song J, Li W, Geistlinger TR, Eeckhoute J. Genome- wide analysis of estrogen receptor binding sites. Nat Genet. 2006;38:1289–97.
Ikeda K, Horie-Inuoe K, Inuoe S. Identification of estrogen-responsive genes based on DNA binding properties of estrogen receptors using high-throughput sequencing technology. Acta Pharmacol Sin. 2015;36:24–31.
Gottardis MM, Robinson SP, Satyaswaroop PG, Jordan VC. Contrasting actions of tamoxifen on endometrial and breast tumor growth in the athymic mouse. Cancer Res. 1988;48:812–5.
Love RR, Mazess RB, Barden HS, et al. Effects of tamoxifen on bone mineral density in postmenopausal women with breast cancer. N Engl J Med. 1992;326:852–6.
McDonnell DP, Wardell SE, Norris JD. Oral selective estrogen receptor downregulators (SERDs), a breakthrough endocrine therapy for breast cancer. J Med Chem. 2015;58:4883–7.
Musgrove EA, Sutherland RL. Biological determinants of endocrine resistance in breast cancer. Nat Rev Cancer. 2009;9:631–43.
Jeselsohn R, Yelensky R, Buchwalter G, et al. Emergence of constitutively active estrogen receptor-α mutations in pretreated advanced estrogen receptor positive breast cancer. Clin Cancer Res. 2014;20:1757–67.
Toy W, Shen Y, Won H, et al. ESR1 ligand binding domain mutations in hormone resistant breast cancer. Nat Genet. 2013;45:1439–45.
Fawell SE, White R, Hoare S, et al. Inhibition of estrogen receptor-DNA binding by the "pure" antiestrogen ICI 164,384 appears to be mediated by impaired receptor dimerization. Proc Natl Acad Sci U S A. 1990;87:6883–7.
Dauvois S, White R, Parker MG. The antiestrogen ICI 182780 disrupts estrogen receptor nucleocytoplasmic shuttling. J Cell Sci. 1993;106:1377–88.
Osborne C, Wakeling A, Nicholson R. Fulvestrant: an oestrogen receptor antagonist with a novel mechanism of action. Br J Cancer. 2004;90:S2–6.
Long X, Nephew KP. Fulvestrant (ICI 182,780)-dependent interacting proteins mediate immobilization and degradation of estrogen receptor. J Biol Chem. 2006;281:9607–15.
Di Leo A, Jerusalem G, Petruzelka L, et al. Results of the CONFIRM Phase III Trial comparing fulvestrant 250 mg with fulvestrant 500 mg in postmenopausal women with estrogen receptor–positive advanced breast cancer. J Clin Oncol. 2010;28:4594–600.
Robertson JF, Lindemann J, Garnett S, et al. A good drug made better: the fulvestrant dose-response story. Clin Breast Cancer. 2014;14:381–9.
Fribbens C, O’Leary B, Kilburn L, et al. Plasma ESR1 mutations and the treatment of estrogen receptor–positive advanced breast cancer. J Clin Oncol. 2016;34:2961–8.
Harrison M, Laight A, Clarke D, et al. Pharmacokinetics and metabolism of fulvestrant after oral, intravenous and intramuscular administration in healthy volunteers. Eur J Cancer Suppl. 2003;1:S171.
Mottamal M, Kang B, Peng X, Wang G. From pure antagonists to pure degraders of the estrogen receptor: evolving strategies for the same target. ACS Omega. 2021;6:9334–43. A mini-review discussing the chemical structure anti-estrogen therapies and the development of oral SERDs.
Bentrem DJ, Dardes RC, Liu H, et al. Molecular mechanism of action at estrogen receptor α of a new clinically relevant antiestrogen (GW7604) related to tamoxifen1. Endocrinology. 2001;142:838–46.
Wu YL, Yang X, Ren Z, McDonnell DP, et al. Structural basis for an unexpected mode of SERM-mediated ER antagonism. Molecular Cell. 2005;18:413–24.
Connor CE, Norris JD, Broadwater G, et al. Circumventing tamoxifen resistance in breast cancers using antiestrogens that induce unique conformational changes in the estrogen receptor. Cancer Res. 2001;61:2917–22.
Dardes RC, O'Regan RM, Gajdos C, et al. Effects of a new clinically relevant antiestrogen (GW5638) related to tamoxifen on breast and endometrial cancer growth in vivo. Clin Cancer Res. 2002;8:1995–2001.
Wardell SE, Ellis MJ, Alley HM, et al. Efficacy of SERD/SERMhybrid-CDK4/6 inhibitor combinations in models of endocrine therapy-resistant breast cancer. Clin Cancer Res. 2015a;21:5121–30.
Garner F, Shomali M, Paquin D, et al. RAD1901: A novel, orally bioavailable selective estrogen receptor degrader that demonstrates antitumor activity in breast cancer xenograft models. Anticancer Drugs. 2015;26:948–56.
Bihani T, Patel HK, Arlt H, et al. Elacestrant (RAD1901), a selective estrogen receptor degrader (SERD), has antitumor activity in multiple ER1 breast cancer patient-derived xenograft models. Clin Cancer Res. 2017;23:4793–804.
Wardell SE, Nelson ER, Chao CA, et al. Evaluation of the pharmacological activities of RAD1901, a selective estrogen receptor degrader. Endocr-Relat Cancer. 2015b;22:713–24.
Bardia A, Kaklamani V, Wilks S, et al. Phase I study of elacestrant (RAD1901), a novel selective estrogen receptor degrader, in ER-Positive, HER2-Negative advanced breast cancer. J Clin Oncol. 2021;39:1360–70.
Bidard F-C, Kaklamani VG, Neven P, et al. Elacestrant (oral selective estrogen receptor degrader) versus standard endocrine therapy for estrogen receptor–positive, human epidermal growth factor receptor 2–negative advanced breast cancer: results from the randomized phase III EMERALD trial. J Clin Oncol. 2022;40(28):3246–56. Results from the first phase III trial of an oral SERD compared to standard of care endocrine therapy in ER+/HER2- MBC.
Sanchez KG, Nangia JR, Schiff R, Rimawi MF. Elacestrant and the promise of oral SERDs. J Clin Oncol. 2022;40(28):3227–9.
Kaklamani VG, Bardia A, Aftimos PG et al. Subgroup analysis of patients with no prior chemotherapy in EMERALD: a phase 3 trial evaluating elacestrant, an oral selective estrogen receptor degrader (SERD), versus investigator’s choice of endocrine monotherapy for ER+/HER2-advanced/metastatic breast cancer (mBC). J Clin Oncol 2021;40(no. 16_suppl):1100.
Metcalfe C, Ingalla E, Blake R, et al. GDC-9545: A novel ER antagonist and clinical candidate that combines desirable mechanistic and pre-clinical DMPK attributes. Cancer Res. 2019;79:P5-04-07. Abstract P5-04-07.
Liang J, Zbieg JR, Blake RA, et al. GDC-9545 (Giredestrant): A potent and orally bioavailable selective estrogen receptor antagonist and degrader with an exceptional preclinical profile for ER+ breast cancer. J Med Chem. 2021;64(16):11841–56.
Jhaveri K, Boni V, Sohn J. Safety and activity of single-agent giredestrant (GDC-9545) from a phase Ia/b study in patients (pts) with estrogen receptorpositive (ER+), HER2-negative locally advanced/metastatic breast cancer (LA/mBC). J Clin Oncol. 2021;39:(no. 15_suppl):1017–7.
Lim E, Jhaveri KL, Perez-Fidalgo JA, et al. A phase Ib study to evaluate the oral selective estrogen receptor degrader GDC- 9545 alone or combined with palbociclib in metastatic ER-positive HER2- negative breast cancer. J Clin Oncol. 2020;38(15_suppl):1023.
Martin JM, Lim E, Chavez MM, et al. Giredestrant (GDC-9545) vs physician choice of endocrine monotherapy (PCET) in patients (pts) with ER+, HER2–locally advanced/metastatic breast cancer (LA/mBC): primary analysis of the phase II, randomised, open-label acelERA BC study. Ann Oncol. 2022;33(suppl_7):S88–S121.
Shomali M, Cheng J, Sun F, et al. SAR439859, a novel selective estrogen receptor degrader (SERD), demonstrates effective and broad antitumor activity in wild-type and mutant ER-positive breast cancer models. Mol Cancer Ther. 2021;20(2):250–62.
Linden HM, Campone M, Bardia A, et al. A phase 1/2 study of amcenestrant (SAR439859), an oral selective estrogen receptor (ER) degrader (SERD), as monotherapy and in combination with other anti-cancer therapies in postmenopausal women with ER-positive (ER+)/human epidermal growth factor receptor 2-negative (HER2–) metastatic breast cancer (mBC): AMEERA-1. Cancer Res. 2021;81(4_suppl):PD8-08.
Tolaney SM, Chan A, Petrakova K, et al. AMEERA-3, a phase II study of amcenestrant (AMC) versus endocrine treatment of physician’s choice (TPC) in patients (pts) with endocrine-resistant ER+/HER2− advanced breast cancer (aBC). Ann Oncol. 2022;33(suppl_7):S88–S121.
Chandarlapaty S, Linden HM, Neven P, et al. AMEERA-1: Phase 1/2 study of amcenestrant (SAR439859), an oral selective estrogen receptor (ER) degrader (SERD), with palbociclib (palbo) in postmenopausal women with ER+/ human epidermal growth factor receptor 2-negative (HER2-) metastatic breast cancer (mBC). J Clin Oncol. 2021;39(no. 15_suppl):1058–8.
Scott JS, Moss T, Stokes S, et al. Abstract 5674: Discovery of AZD9833, an oral small molecule selective degrader of the estrogen receptor (SERD). Cancer Res. 2020;80(16_suppl):5674.
Baird R, Oliveira M, Ciruelos EM, et al. Updated data from SERENA-1: a phase 1 dose escalation and expansion study of the next generation oral SERD AZD9833 as a monotherapy and in combination with palbociclib, in women with ER-positive, HER2-negative advanced breast cancer. Cancer Res. 2021;81(4_Suppl):PS11-05.
Oliveira M, Hamilton EP, Incorvati J et al. Serena-1: Updated analyses from a phase 1 study (parts C/D) of the next-generation oral SERD camizestrant (AZD9833) in combination with palbociclib, in women with ER-positive, HER2-negative advanced breast cancer. Journal of Clinical Oncology 2022;40(no. 16_suppl):1032.
Oliveira M, Pominchuk D, Nowecki Z, et al. Camizestrant, a next-generation oral SERD vs fulvestrant in post-menopausal women with advanced ER-positive HER2-negative breast cancer: results of the randomized, multi-dose phase 2 SERENA-2 trial. Presented at SABCS 2022. December 6-10, 2022. Abstract GS3-02. Randomized phase 2 data of a novel oral SERD vs standard endocrine therapy in ER+/HER2- MBC.
Jhaveri K, Juric D, Yap Y-S, et al. A phase I study of LSZ102, an oral selective estrogen receptor degrader, with or without ribociclib or alpelisib, in patients with estrogen receptor–positive breast cancer. Clin Cancer Res. 2021;27(21):5760–70.
Aftimos P, Neven P, Pegram M, et al. Rintodestrant (G1T48), an oral selective estrogen receptor degrader in ER+/HER2- locally advanced or metastatic breast cancer: updated phase 1 results and dose selection. Cancer Res. 2021;81(4 suppl):Abstract nr PS12-04.
Kalinksy K, Abramson V, Chalasani P, et al. ZN-c5, an oral selective estrogen receptor degrader (SERD), in women with advanced estrogen receptor-positive (ER+)/human epidermal growth factor receptor 2 negative (HER2-) breast cancer. Cancer Res. 2022;82(4_suppl):P1-17-02.
Bidard FC, Hardy-Bessard AC, Dalenc F, et al. Switch to fulvestrant and palbociclib versus no switch in advanced breast cancer with rising ESR1 mutation during aromatase inhibitor and palbociclib therapy (PADA-1): a randomised, open-label, multicentre, phase 3 trial. Lancet Oncol. 2022;23(11):1367–77. First proof of concept trial that demonstrated the benefit of utilizing plasma ctDNA monitoring to guide treatment strategy in ER+/HER2-MBC.
Di Leo A, Jerusalem G, Petruzelka L, et al. Results of the CONFIRM phase III trial comparing fulvestrant 250 mg with fulvestrant 500 mg in postmenopausal women with estrogen receptor-positive advanced breast cancer. J Clin Oncol. 2010;28(30):4594–600.
Hurvitz SA, Park YH, Bardia A et al. LBA14 Neoadjuvant giredestrant (GDC-9545) + palbociclib (palbo) vs anastrozole (A) + palbo in post-menopausal women with oestrogen receptor-positive, HER2-negative, untreated early breast cancer (ER+/HER2– eBC): Interim analysis of the randomised, open-label, phase II coopERA BC study Ann Oncol 2021;32:S1285–6.
Campone M, Dong Y, Ling B, et al. AMEERA-4: a preoperative window-of-opportunity (WOO) study to assess the pharmacodynamic (PD) activity of amcenestrant or letrozole in postmenopausal patients with ER+/HER2− primary breast cancer. J Clin Oncol 2022;40(no. 16_suppl):528−8.
Komm BS, Chines AA. An update on selective estrogen receptor modulators for the prevention and treatment of osteoporosis. Maturitas. 2012;71(3):221–6.
LaCroix AZ, Powles T, Osborne CK, et al. Breast cancer incidence in the randomized pearl trial of lasofoxifene in postmenopausal osteoporotic women. JNCI Journal of the National Cancer Institute. 2010;102:1706–15.
Gardner M, Taylor A, Wei G, et al. Clinical pharmacology of multiple doses of lasofoxifene in postmenopausal women. J Clin Pharmacol. 2006;46:52–8.
Laine M, Greene M, Chang Y-F, et al. Lasofoxifene decreases breast cancer lung and liver metastasis in a mammary intraductal (MIND) xenograft model of mutant ERα+ breast cancer. Cancer Res. 2019;79(4 Suppl): Abstract nr PD7-09.
Goetz M, Plourde P, Stover DG, et al. Open-label, randomized study of lasofoxifene (LAS) vs fulvestrant (Fulv) for women with locally advanced/metastatic ER+/HER2- breast cancer (mBC), an estrogen receptor 1 (ESR1) mutation, and disease progression on aromatase (AI) and cyclindependent kinase 4/6 (CDK4/6i) inhibitors. Ann Oncol. 2022;33:S1387–8.
Damodaran S, Plourde PV, Moore HCF, et al. Open-label, phase 2, multicenter study of lasofoxifene (LAS) combined with abemaciclib (Abema) for treating pre- and postmenopausal women with locally advanced or metastatic ER+/HER2− breast cancer and an ESR1 mutation after progression on prior therapies. J Clin Oncol 2022;40(no. 16_suppl):1022. Preliminary data showing that combination of a novel SERM and CDK 4/6 inhibitor improves median PFS in ESR1 mutant ER+/HER2- MBC after progression on prior CDK 4/6i and fulvestrant.
Békés M, Langley DR, Crews CM. PROTAC targeted protein degraders: the past is prologue. Nat Rev Drug Discov. 2022;21:181–200.
Hamilton E, Vahdat L, Han HS, et al. First-in-human safety and activity of ARV-471, a novel PROTAC® estrogen receptor degrader, in ER+/HER2- locally advanced or metastatic breast cancer. Cancer Res. 2022;82(4 suppl): Abstract nr PD13-08.
Hurvitz S, Schott AF, Ma C et al. ARV-471, a PROTAC® estrogen receptor (ER) degrader in advanced ER-positive/human epidermal growth factor receptor 2 (HER2)-negative breast cancer: phase 2 expansion (VERITAC) of a phase 1/2 study. Presented at SABCS 2022. December 6-10, 2022. Abstract GS3-03.
He W, Zhang H, Perkins L et al. Abstract PS18-09: novel chimeric small molecule AC682 potently degrades estrogen receptor with oral anti-tumor efficacy superior to fulvestrant. Cancer Res. 2021;81(4_suppl): PS18 09.
Korpal M, Puyang X, Furman C, et al. Development of a first-in-class oral selective ERα covalent antagonist (SERCA) for the treatment of ERαWT and ERαMUT breast cancer. Cancer Res. 2018;78(4 suppl): Abstract nr P1-10-08.
Puyang X, Furman C, Banka D, et al. Discovery of selective estrogen receptor covalent antagonists for the treatment of ER alpha WT and ER alpha MUT breast cancer. Cancer Discov. 2018;8:1176–93.
Hamilton EP, Wang JS, Pluard T, et al. Phase I/II trial of H3B-6545, a novel selective estrogen receptor covalent antagonist (SERCA), in estrogen receptor positive (ER+), human epidermal growth factor receptor 2 negative (HER2-)advanced breast cancer. Cancer Res. 2021;81(4 suppl): Abstract nr PD8-06.
Hodges-Gallagher L, Sun R, Myles D, et al. OP-1250: a potent orally available complete antagonist of estrogen receptor-mediated signaling that shrinks wild type and mutant breast tumors. Eur J Cancer. 2020;138:S55.
Patel M, Alemany C, Mitri Z et al Preliminary data from a phase I/II, multicenter, dose escalation study of OP-1250, an oral CERAN/SERD, in subjects with advanced and/or metastatic estrogen receptor (ER)-positive, HER2-negative breast cancerCancer Res 2022;82 (4_suppl):P1-17-12.
Alemany C, Patel M, Mitri Z, et al. A phase 1/2 dose escalation and expansion study of OP-1250 in adults with advanced and/or metastatic hormone receptorpositive (HR+), HER2-negative (HER2-) Breast Cancer (NCT04505826). Mol Cancer Ther. 2021;20(12_suppl):P037.
Hamilton E, Meisel J, Alemany C, et al. Phase 1b results from OP-1250-001, a dose escalation and dose expansion study of OP-1250, an oral CERAN, in subjects with advanced and/or metastatic estrogen receptor (ER)-positive, HER2-negative breast cancer (NCT04505826). Eur J Cancer. 2022;174:S36.
Osborne C, Richards DA, Wilks ST et al. SABCS 2020 A phase 1 study of D-0502, an orally bioavailable SERD, for advanced or metastatic HR-positive and HER2-negative breast cancer. Cancer Res 2021;81(4 suppl): Abstract nr PS11-26.
Jhaveri K, Jeselsohn R, Lim E et al. A phase 1a/b trial of imlunestrant (LY3484356), an oral selective estrogen receptor degrader (SERD) in ER-positive (ER+) advanced breast cancer (aBC) and endometrial endometrioid cancer (EEC): monotherapy results from EMBER. J Clin Oncol 2022;40(no. 16_suppl):1021.
Maglakelidze M, Bulat I, Ryspayeva D, et al. Rintodestrant (G1T48), an oral selective estrogen receptor degrader, in combination with palbociclib for ER+/HER2– advanced breast cancer: phase 1 results. J Clin Oncol 2021;39(no. 15_suppl):1063.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Mythili Shastry reports no conflict of interest.
Erika Hamilton’s institution has received research funding from multiple entities (see below).
AbbVie | Research funding—paid to institution |
|---|---|
Acerta Pharma | Research funding—paid to institution |
Accutar Biotechnology | Research funding—paid to institution |
ADC Therapeutics | Research funding—paid to institution |
AKESOBIO Australia | Research funding—paid to institution |
Amgen | Research funding—paid to institution |
Aravive | Research funding—paid to institution |
ArQule | Research funding—paid to institution |
Artios | Research funding—paid to institution |
Arvinas | Research funding—paid to institution |
Astra Zeneca | Research funding—paid to institution |
AtlasMedx | Research funding—paid to institution |
BeiGene | Research funding—paid to institution |
Black Diamond | Research funding—paid to institution |
Bliss BioPharmaceuticals | Research funding—paid to institution |
Boehringer Ingelheim | Research funding—paid to institution |
Cascadian Therapeutics | Research funding—paid to institution |
Clovis | Research funding—paid to institution |
Compugen | Research funding—paid to institution |
Context Therapeutics | Research funding—paid to institution |
Cullen-Florentine | Research funding—paid to institution |
Curis | Research funding—paid to institution |
CytomX | Research funding—paid to institution |
Daiichi Sankyo | Research funding—paid to institution |
Dana Farber Cancer Inst | Research funding—paid to institution |
Dantari | Research funding—paid to institution |
Deciphera | Research funding—paid to institution |
Duality Biologics | Research funding—paid to institution |
eFFECTOR Therapeutics | Research funding—paid to institution |
Ellipses Pharma | Research funding—paid to institution |
Elucida Oncology | Research funding—paid to institution |
EMD Serono | Research funding—paid to institution |
Fujifilm | Research funding—paid to institution |
G1 Therapeutics | Research funding—paid to institution |
H3 Biomedicine | Research funding—paid to institution |
Harpoon | Research funding—paid to institution |
Hutchison MediPharma | Research funding—paid to institution |
Immunogen | Research funding—paid to institution |
Immunomedics | Research funding—paid to institution |
Incyte | Research funding—paid to institution |
Infinity Pharmaceuticals | Research funding—paid to institution |
InventisBio | Research funding—paid to institution |
Jacobio | Research funding—paid to institution |
K-Group Beta | Research funding—paid to institution |
Karyopharm | Research funding—paid to institution |
Kind Pharmaceuticals | Research funding—paid to institution |
Leap Therapeutics | Research funding—paid to institution |
Lilly | Research funding—paid to institution |
Loxo Oncology | Research funding—paid to institution |
Lycera | Research funding—paid to institution |
MabSpace Biosciences | Research funding—paid to institution |
MacroGenics | Research funding—paid to institution |
MedImmune | Research funding—paid to institution |
Mersana | Research funding—paid to institution |
Merus | Research funding—paid to institution |
Millennium | Research funding—paid to institution |
Molecular Templates | Research funding—paid to institution |
Novartis | Research funding—paid to institution |
NuCana | Research funding—paid to institution |
Olema | Research funding—paid to institution |
OncoMed | Research funding—paid to institution |
Onconova Therapeutics | Research funding—paid to institution |
Oncothyreon | Research funding—paid to institution |
ORIC Pharmaceuticals | Research funding—paid to institution |
Orinove | Research funding—paid to institution |
Orum Therapeutics | Research funding—paid to institution |
Pfizer | Research funding—paid to institution |
PharmaMar | Research funding—paid to institution |
Pieris Pharmaceuticals | Research funding—paid to institution |
Pionyr Immunotherapeutics | Research funding—paid to institution |
Plexxikon | Research funding—paid to institution |
Prelude Therapeutics | Research funding—paid to institution |
Profound Bio | Research funding—paid to institution |
Radius Health | Research funding—paid to institution |
Regeneron | Research funding—paid to institution |
Relay Therapeutics | Research funding—paid to institution |
Repertoire Immune Medicine | Research funding—paid to institution |
Rgenix | Research funding—paid to institution |
Roche/Genentech | Research funding—paid to institution |
Seagen | Research funding—paid to institution |
Sermonix Pharmaceuticals | Research funding—paid to institution |
Shattuck Labs | Research funding—paid to institution |
StemCentRx | Research funding—paid to institution |
Sutro | Research funding—paid to institution |
Syndax | Research funding—paid to institution |
Syros | Research funding—paid to institution |
Taiho | Research funding—paid to institution |
TapImmune | Research funding—paid to institution |
Tesaro | Research funding—paid to institution |
Tolmar | Research funding—paid to institution |
Torque Therapeutics | Research funding—paid to institution |
Treadwell Therapeutics | Research funding—paid to institution |
Verastem | Research funding—paid to institution |
Vincerx Pharma | Research funding—paid to institution |
Zenith Epigenetics | Research funding—paid to institution |
Zymeworks | Research funding—paid to institution |
Erika Hamilton’s institution has received consulting fees from multiple entities (see below).
Time frame: past 36 months | |
|---|---|
Arcus | Consulting—paid to institution |
Astra Zeneca | Consulting—paid to institution |
Daiichi Sankyo | Consulting—paid to institution |
Ellipses Pharma | Consulting—paid to institution |
Greenwich LifeSciences | Consulting—paid to institution |
iTeos | Consulting—paid to institution |
Janssen | Consulting—paid to institution |
Lilly | Consulting—paid to institution |
Loxo | Consulting—paid to institution |
Mersana | Consulting—paid to institution |
Novartis | Consulting—paid to institution |
Olema Pharmaceuticals | Consulting—paid to institution |
Orum Therapeutics | Consulting—paid to institution |
Pfizer | Consulting—paid to institution |
Relay Therapeutics | Consulting—paid to institution |
Roche/Genentech | Consulting—paid to institution |
Seagen | Consulting—paid to institution |
Stemline Therapeutics | Consulting—paid to institution |
Tubulis | Consulting—paid to institution |
Verascity Science | Consulting—paid to institution |
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shastry, M., Hamilton, E. Novel Estrogen Receptor-Targeted Agents for Breast Cancer. Curr. Treat. Options in Oncol. 24, 821–844 (2023). https://doi.org/10.1007/s11864-023-01079-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11864-023-01079-y