Opinion Statement
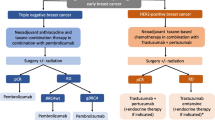
Treatment sequencing in early-stage breast cancer has significantly evolved in recent years, particularly in the triple negative (TNBC) and human epidermal growth factor receptor 2 (HER2)-positive subsets. Instead of surgery first followed by chemotherapy, several clinical trials showed benefits to administering systemic chemotherapy (and HER2-targeted therapies) prior to surgery. These benefits include more accurate prognostic estimates based on the extent of residual cancer that can also guide adjuvant treatment, and frequent tumor downstaging that can lead to smaller surgeries in patients with large tumors at diagnosis. Patients with extensive invasive residual cancer after neoadjuvant therapy are at high risk for disease recurrence, and two pivotal clinical trials, CREATE-X and KATHERINE, demonstrated improved recurrence free survival with adjuvant capecitabine and ado-trastuzumab-emtansine (T-DM1) in TNBC and HER2-positive residual cancers, respectively. Patients who achieve pathologic complete response (pCR) have excellent long-term disease-free survival regardless of what chemotherapy regimen induced this favorable response. This allows escalation or de-escalation of adjuvant therapy: patients who achieved pCR could be spared further chemotherapy, while those with residual cancer could receive additional chemotherapy postoperatively. Ongoing clinical trials are testing this strategy (CompassHER2-pCR: NCT04266249). pCR also provides an opportunity to assess de-escalation of locoregional therapies. Currently, for patients with residual disease in the lymph nodes (ypN+), radiation therapy entails coverage of the undissected axilla, and may include supra/infraclavicular/internal mammary nodes in addition to the whole breast or chest wall, depending on the type of surgery. Ongoing trials are testing the safety of omitting post-mastectomy breast and post-lumpectomy nodal irradiation (NCT01872975) as well as omitting axillary lymph node dissection (NCT01901094) in the setting of pCR. Additionally, evolving technologies such as minimal residual disease (MRD) monitoring in the blood during follow-up may allow early intervention with “second-line systemic adjuvant therapy” for patients with molecular relapse which might prevent impending clinical relapse.
Similar content being viewed by others
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Asselain B, Barlow W, Bartlett J, Bergh J, Bergsten-Nordström E, Bliss J, et al. Long-term outcomes for neoadjuvant versus adjuvant chemotherapy in early breast cancer: meta-analysis of individual patient data from ten randomised trials. Lancet Oncol. 2018;19(1):27–39.
Mauri D, Pavlidis N, Ioannidis JPA. Neoadjuvant versus adjuvant systemic treatment in breast cancer: a meta-analysis. J Natl Cancer Inst. 2005;97(3):188–94.
Ragaz J, Baird R, Rebbeck P, Trevisan C, Goldie J, Coldman A, et al. Preoperative (neoadjuvant) versus postoperative adjuvant chemotherapy for stage I–II breast cancer. Long-term analysis of British Columbia randomized trial. Proc Am Soc Clin Oncol. 1997;16:142a.
Wolmark N, Wang J, Mamounas E, Bryant J, Fisher B. Preoperative chemotherapy in patients with operable breast cancer: nine-year results from National Surgical Adjuvant Breast and Bowel Project B-18. J Natl Cancer Inst Monogr. 2001;30:96–102.
Fisher B, Bryant J, Wolmark N, Mamounas E, Brown A, Fisher ER, et al. Effect of preoperative chemotherapy on the outcome of women with operable breast cancer. J Clin Oncol. 1998;16(8):2672–85.
Boughey JC, Suman VJ, Mittendorf EA, Ahrendt GM, Wilke LG, Taback B, et al. Sentinel lymph node surgery after neoadjuvant chemotherapy in patients with node-positive breast cancer: the ACOSOG Z1071 (Alliance) clinical trial. JAMA. 2013;310(14):1455–61.
Kuehn T, Bauerfeind I, Fehm T, Fleige B, Hausschild M, Helms G, et al. Sentinel-lymph-node biopsy in patients with breast cancer before and after neoadjuvant chemotherapy (SENTINA): a prospective, multicentre cohort study. Lancet Oncol. 2013;14(7):609–18.
Boileau J-F, Poirier B, Basik M, Holloway CMB, Gaboury L, Sideris L, et al. Sentinel node biopsy after neoadjuvant chemotherapy in biopsy-proven node-positive breast cancer: the SN FNAC study. J Clin Oncol. 2015;33(3):258–64.
Mamounas EP, Kuehn T. Rutgers EJT. Lancet: von Minckwitz G. Current approach of the axilla in patients with early-stage breast cancer; 2017.
Hunt KK, Yi M, Mittendorf EA, Guerrero C, Babiera GV, Bedrosian I, et al. Sentinel lymph node surgery after neoadjuvant chemotherapy is accurate and reduces the need for axillary dissection in breast cancer patients. Ann Surg. 2009;250(4):558–64.
Boughey JC, Peintinger F, Meric-Bernstam F, Perry AC, Hunt KK, Babiera GV, et al. Impact of preoperative versus postoperative chemotherapy on the extent and number of surgical procedures in patients treated in randomized clinical trials for breast cancer. Ann Surg. 2006;244(3):464–70.
Gianni L, Pienkowski T, Im Y-H, Roman L, Tseng L-M, Liu M-C, et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial. Lancet Oncol. 2012;13(1):25–32.
Gianni L, Pienkowski T, Im Y-H, Tseng L-M, Liu M-C, Lluch A, et al. 5-year analysis of neoadjuvant pertuzumab and trastuzumab in patients with locally advanced, inflammatory, or early-stage HER2-positive breast cancer (NeoSphere): a multicentre, open-label, phase 2 randomised trial. Lancet Oncol. 2016;17(6):791–800.
von Minckwitz G, Untch M, Blohmer J-U, Costa SD, Eidtmann H, Fasching PA, et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol. 2012;30(15):1796–804.
Schneeweiss A, Chia S, Hickish T, Harvey V, Eniu A, Hegg R, et al. Pertuzumab plus trastuzumab in combination with standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer: a randomized phase II cardiac safety study (TRYPHAENA). Ann Oncol. 2013;24(9):2278–84.
Schmid P, Cortes J, Pusztai L, McArthur H, Kümmel S, Bergh J, et al. Pembrolizumab for early triple-negative breast cancer. N Engl J Med. 2020;382(9):810–21.
Sikov WM, Berry DA, Perou CM, Singh B, Cirrincione CT, Tolaney SM, et al. Impact of the addition of carboplatin and/or bevacizumab to neoadjuvant once-per-week paclitaxel followed by dose-dense doxorubicin and cyclophosphamide on pathologic complete response rates in stage II to III triple-negative breast cancer: CALGB 40603 (Alliance). J Clin Oncol. 2015;33(1):13–21.
Loibl S, O’Shaughnessy J, Untch M, Sikov WM, Rugo HS, McKee MD, et al. Addition of the PARP inhibitor veliparib plus carboplatin or carboplatin alone to standard neoadjuvant chemotherapy in triple-negative breast cancer (BrighTNess): a randomised, phase 3 trial. Lancet Oncol. 2018;19(4):497–509.
Green MC, Buzdar AU, Smith T, Ibrahim NK, Valero V, Rosales MF, et al. Weekly paclitaxel improves pathologic complete remission in operable breast cancer when compared with paclitaxel once every 3 weeks. J Clin Oncol. 2005;23(25):5983–92.
Llombart-Cussac A, Bermejo B, Villanueva C, Delaloge S, Morales S, Balmaña J, et al. SOLTI NeoPARP: a phase II randomized study of two schedules of iniparib plus paclitaxel versus paclitaxel alone as neoadjuvant therapy in patients with triple-negative breast cancer. Breast Cancer Res Treat. 2015;154(2):351–7.
Foldi J, Silber A, Reisenbichler E, Singh K, Fischbach N, Persico J, et al. Neoadjuvant durvalumab plus weekly nab-paclitaxel and dose-dense doxorubicin/cyclophosphamide in triple-negative breast cancer. NPJ Breast Cancer. 2021;7(1):9.
Foldi J, Mougalian S, Silber A, Lannin D, Killelea B, Chagpar A, et al. Single-arm, neoadjuvant, phase II trial of pertuzumab and trastuzumab administered concomitantly with weekly paclitaxel followed by 5-fluoruracil, epirubicin, and cyclophosphamide (FEC) for stage I-III HER2-positive breast cancer. Breast Cancer Res Treat. 2018;169(2):333–40.
Weiss A, Bashour SI, Hess K, Thompson AM, Ibrahim NK. Effect of neoadjuvant chemotherapy regimen on relapse-free survival among patients with breast cancer achieving a pathologic complete response: an early step in the de-escalation of neoadjuvant chemotherapy. Breast Cancer Res BCR. 2018;20(1):27.
Yee D, Demichele AM, Yau C, Isaacs C, Symmans WF, Albain KS, et al. Association of event-free and distant recurrence-free survival with individual-level pathologic complete response in neoadjuvant treatment of stages 2 and 3 breast cancer: three-year follow-up analysis for the I-SPY2 adaptively randomized clinical trial. JAMA Oncol. 2020;6(9):1355–62.
Symmans WF, Wei C, Gould R, Yu X, Zhang Y, Liu M, et al. Long-term prognostic risk after neoadjuvant chemotherapy associated with residual cancer burden and breast cancer subtype. J Clin Oncol. 2017;35(10):1049–60.
Liedtke C, Mazouni C, Hess KR, André F, Tordai A, Mejia JA, et al. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J Clin Oncol. 2008;26(8):1275–81.
Carey LA, Metzger R, Dees EC, Collichio F, Sartor CI, Ollila DW, et al. American Joint Committee on Cancer tumor-node-metastasis stage after neoadjuvant chemotherapy and breast cancer outcome. J Natl Cancer Inst. 2005;97(15):1137–42.
Symmans WF, Peintinger F, Hatzis C, Rajan R, Kuerer H, Valero V, et al. Measurement of residual breast cancer burden to predict survival after neoadjuvant chemotherapy. J Clin Oncol. 2007;25(28):4414–22.
Cortazar P, Zhang L, Untch M, Mehta K, Costantino JP, Wolmark N, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384(9938):164–72.
Mittendorf EA, Vila J, Tucker SL, Chavez-MacGregor M, Smith BD, Symmans WF, et al. The Neo-Bioscore update for staging breast cancer treated with neoadjuvant chemotherapy. JAMA Oncol. 2016;2(7):929–36.
Marmé F, Lederer B, Blohmer J-U, Costa SD, Denkert C, Eidtmann H, et al. Utility of the CPS+EG staging system in hormone receptor-positive, human epidermal growth factor receptor 2-negative breast cancer treated with neoadjuvant chemotherapy. Eur J Cancer 1990. 2016;53:65–74.
van der Noordaa ME, Yau C, Shad S, Osdoit M, Steenbruggen TG, de Croze D, et al. Abstract GS4-07: assessing prognosis after neoadjuvant therapy: a comparison between anatomic ypAJCC staging, residual cancer burden class and neo-bioscore. Cancer Res. 2021;81(4 Supplement):GS4-GS4-07.
Nanda R, Liu MC, Yau C, Shatsky R, Pusztai L, Wallace A, et al. Effect of pembrolizumab plus neoadjuvant chemotherapy on pathologic complete response in women with early-stage breast cancer: an analysis of the ongoing phase 2 adaptively randomized I-SPY2 trial. JAMA Oncol. 2020.
Pusztai L, Foldi J, Dhawan A, DiGiovanna MP, Mamounas EP. Changing frameworks in treatment sequencing of triple-negative and HER2-positive, early-stage breast cancers. Lancet Oncol. 2019;20(7):e390–6.
•• Masuda N, Lee S-J, Ohtani S, Im Y-H, Lee E-S, Yokota I, et al. Adjuvant capecitabine for breast cancer after preoperative chemotherapy. N Engl J Med. 2017;376(22):2147–59 Phase III randomized trial (CREATE-X) of adjuvant capecitabine versus observation in patients with HER2-negative breast cancer who had residual disease after neoadjuvant chemotherapy containing an antrhracycline, taxane, or both.
Zujewski JA, Rubinstein L. CREATE-X a role for capecitabine in early-stage breast cancer: an analysis of available data. NPJ Breast Cancer. 2017;3:27.
Zhang Z-C, Xu Q-N, Lin S-L, Li X-Y. Capecitabine in combination with standard (neo)adjuvant regimens in early breast cancer: survival outcome from a meta-analysis of randomized controlled trials. PLoS One. 2016;11(10):e0164663.
• Wang X, Wang S-S, Huang H, Cai L, Zhao L, Peng R-J, et al. Effect of capecitabine maintenance therapy using lower dosage and higher frequency vs observation on disease-free survival among patients with early-stage triple-negative breast cancer who had received standard treatment: the SYSUCC-001 randomized clinical trial. JAMA. 2021;325(1):50–8 Phase III randomized trial in early-stage TNBC patients who had received standard adjuvant or neoadjuvant chemotherapy evaluating 1 year of metronomic (uninterrupted administration) capecitabine versus observation.
IDMC has concluded that OlympiA trial of Lynparza crossed superiority boundary for invasive disease-free survival vs. placebo at planned interim analysis [Internet]. [cited 2021 Mar 29]. Available from: https://www.astrazeneca.com/media-centre/press-releases/2021/olympia-trial-of-lynparza-idmc-recommend-early-analysis.html
•• von Minckwitz G, Huang C-S, Mano MS, Loibl S, Mamounas EP, Untch M, et al. Trastuzumab emtansine for residual invasive HER2-positive breast cancer. N Engl J Med. 2019;380(7):617–28 Phase III randomized trial (KATHERINE) of adjuvant ado-trastuzumab-emtansine (T-DM1) versus trastuzumab in patients with HER2-positive breast cancer who had residual disease after neoadjuvant therapy containing trastuzumab.
Chan A, Delaloge S, Holmes FA, Moy B, Iwata H, Harvey VJ, et al. Neratinib after trastuzumab-based adjuvant therapy in patients with HER2-positive breast cancer (ExteNET): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2016;17(3):367–77.
• Chan A, Moy B, Mansi J, Ejlertsen B, Holmes FA, Chia S, et al. Final efficacy results of neratinib in HER2-positive hormone receptor-positive early-stage breast cancer from the phase III ExteNET trial. Clin Breast Cancer. 2021;21(1):80–91.e7 Phase III randomized placebo-controlled trial of 1 year of neratinib versus placebo added to adjuvant endocrine therapy in patients with HER2- and ER-positive early breast cancer who received adjuvant or neoadjuvant chemotherapy and trastuzumab.
. von Minckwitz G, Procter M, de Azambuja E, Zardavas D, Benyunes M, Viale G, et al. Adjuvant pertuzumab and trastuzumab in early HER2-positive breast cancer. N Engl J Med. 2017;377(2):122–31 Phase III randomized, placebo-controlled trial of adjuvant pertuzumab or placebo added to trastuzumab following standard adjuvant chemotherapy for high-risk early-stage HER2-positive breast cancer.
Perez EA, Barrios C, Eiermann W, Toi M, Im Y-H, Conte P, et al. Trastuzumab emtansine with or without pertuzumab versus trastuzumab plus taxane for human epidermal growth factor receptor 2-positive, advanced breast cancer: primary results from the phase III MARIANNE study. J Clin Oncol. 2017;35(2):141–8.
Dzimitrowicz H, Berger M, Vargo C, Hood A, Abdelghany O, Raghavendra AS, et al. T-DM1 activity in metastatic human epidermal growth factor receptor 2-positive breast cancers that received prior therapy with trastuzumab and pertuzumab. J Clin Oncol. 2016;34(29):3511–7.
Johnston SRD, Harbeck N, Hegg R, Toi M, Martin M, Shao ZM, et al. Abemaciclib combined with endocrine therapy for the adjuvant treatment of HR+, HER2-, node-positive, high-risk, early breast cancer (monarchE). J Clin Oncol. 2020;38(34):3987–98.
Loibl S, Marmé F, Martin M, Untch M, Bonnefoi H, Kim S-B, et al. Abstract GS1-02: Phase III study of palbociclib combined with endocrine therapy (ET) in patients with hormone-receptor-positive (HR+), HER2-negative primary breast cancerand with high relapse risk after neoadjuvant chemotherapy (NACT): first results from PENELOPE-B. Cancer Res. 2021;81(4 Supplement):GS1-GS1-02.
Mayer EL, Dueck AC, Martin M, Rubovszky G, Burstein HJ, Bellet-Ezquerra M, et al. Palbociclib with adjuvant endocrine therapy in early breast cancer (PALLAS): interim analysis of a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2021;22(2):212–22.
Goldenberg DM, Stein R, Sharkey RM. The emergence of trophoblast cell-surface antigen 2 (TROP-2) as a novel cancer target. Oncotarget. 2018;9(48):28989–9006.
Bardia A, Mayer IA, Vahdat LT, Tolaney SM, Isakoff SJ, Diamond JR, et al. Sacituzumab Govitecan-hziy in refractory metastatic triple-negative breast cancer. N Engl J Med. 2019;380(8):741–51.
Modi S, Saura C, Yamashita T, Park YH, Kim S-B, Tamura K, et al. Trastuzumab deruxtecan in previously treated HER2-positive breast cancer. N Engl J Med. 2020;382(7):610–21.
Modi S, Tsurutani J, Tamura K, Park H, Sagara Y, Murthy R, et al. Abstract P6-17-02: trastuzumab deruxtecan (DS-8201a) in subjects with HER2-low expressing breast cancer: updated results of a large phase 1 study. Cancer Res 2019;79(4 Supplement):P6-17-02-P6-17–02.
•• Radovich M, Jiang G, Hancock BA, Chitambar C, Nanda R, Falkson C, et al. Association of circulating tumor DNA and circulating tumor cells after neoadjuvant chemotherapy with disease recurrence in patients with triple-negative breast cancer: preplanned secondary analysis of the BRE12-158 randomized clinical trial. JAMA Oncol. 2020;6(9):1410–5 A study using tissues collected from patients enrolled in the BRE12-158 clinical trial to evaluate whether the presence of ctDNA and CTCs after neoadjuvant chemotherapy in patients with early-stage TNBC is associated with clinical outcomes.
Schneider B, Miller KD, Badve S, O’Neil B, Helft P, Chitambar C, et al. Abstract OT3-04-01: BRE12-158: a phase II randomized controlled trial of genomically directed therapy after preoperative chemotherapy in patients with triple negative breast cancer (TNBC). Cancer Res. 2017;77(4 Supplement):OT3-04-01-OT3-04–01.
Garcia-Murillas I, Schiavon G, Weigelt B, Ng C, Hrebien S, Cutts RJ, et al. Mutation tracking in circulating tumor DNA predicts relapse in early breast cancer. Sci Transl Med. 2015;7(302):302ra133.
Neumann MHD, Bender S, Krahn T. Schlange T. ctDNA and CTCs in liquid biopsy - current status and where we need to progress. Comput Struct Biotechnol J. 2018;16:190–5.
Chen Y-H, Hancock BA, Solzak JP, Brinza D, Scafe C, Miller KD, et al. Next-generation sequencing of circulating tumor DNA to predict recurrence in triple-negative breast cancer patients with residual disease after neoadjuvant chemotherapy. NPJ Breast Cancer. 2017;3:24.
Huang EH, Tucker SL, Strom EA, McNeese MD, Kuerer HM, Buzdar AU, et al. Postmastectomy radiation improves local-regional control and survival for selected patients with locally advanced breast cancer treated with neoadjuvant chemotherapy and mastectomy. J Clin Oncol Off J Am Soc Clin Oncol. 2004;22(23):4691–9.
McGuire SE, Gonzalez-Angulo AM, Huang EH, Tucker SL, Kau S-WC YT-K, et al. Postmastectomy radiation improves the outcome of patients with locally advanced breast cancer who achieve a pathologic complete response to neoadjuvant chemotherapy. Int J Radiat Oncol Biol Phys. 2007;68(4):1004–9.
Fisher B, Brown A, Mamounas E, Wieand S, Robidoux A, Margolese RG, et al. Effect of preoperative chemotherapy on local-regional disease in women with operable breast cancer: findings from National Surgical Adjuvant Breast and Bowel Project B-18. J Clin Oncol. 1997;15(7):2483–93.
Bear HD, Anderson S, Brown A, Smith R, Mamounas EP, Fisher B, et al. The effect on tumor response of adding sequential preoperative docetaxel to preoperative doxorubicin and cyclophosphamide: preliminary results from National Surgical Adjuvant Breast and Bowel Project Protocol B-27. J Clin Oncol. 2003;21(22):4165–74.
Boughey JC, Ballman KV, Le-Petross HT, McCall LM, Mittendorf EA, Ahrendt GM, et al. Identification and resection of clipped node decreases the false-negative rate of sentinel lymph node surgery in patients presenting with node-positive breast cancer (T0-T4, N1-N2) who receive neoadjuvant chemotherapy: results from ACOSOG Z1071 (Alliance). Ann Surg. 2016;263(4):802–7.
Caudle AS, Yang WT, Krishnamurthy S, Mittendorf EA, Black DM, Gilcrease MZ, et al. Improved axillary evaluation following neoadjuvant therapy for patients with node-positive breast cancer using selective evaluation of clipped nodes: implementation of targeted axillary dissection. J Clin Oncol. 2016;34(10):1072–8.
Moo T-A, Edelweiss M, Hajiyeva S, Stempel M, Raiss M, Zabor EC, et al. Is low-volume disease in the sentinel node after neoadjuvant chemotherapy an indication for axillary dissection? Ann Surg Oncol. 2018;25(6):1488–94.
Barron AU, Hoskin TL, Boughey JC. Predicting non-sentinel lymph node metastases in patients with a positive sentinel lymph node after neoadjuvant chemotherapy. Ann Surg Oncol. 2018;25(10):2867–74.
Wong SM, Almana N, Choi J, Hu J, Gagnon H, Natsuhara K, et al. Prognostic significance of residual axillary nodal micrometastases and isolated tumor cells after neoadjuvant chemotherapy for breast cancer. Ann Surg Oncol. 2019 Oct;26(11):3502–9.
Wong SM, Weiss A, Mittendorf EA, King TA, Golshan M. Surgical management of the axilla in clinically node-positive patients receiving neoadjuvant chemotherapy: a national cancer database analysis. Ann Surg Oncol. 2019;26(11):3517–25.
Weiss A, Campbell J, Ballman KV, Sikov W, Carey L, Ollila DW. Factors associated with residual nodal disease among breast cancer patients treated with neoadjuvant chemotherapy: results of CALGB 40601 (HER2+) and 40603 (triple-negative) (Alliance). Present Soc Surg Oncol Annu Meet. 2021;2021.
Yau C, van der Noordaa M, Wei J, Osdoit M, Reyal F, Hamy A-S, et al. Abstract GS5-01: Residual cancer burden after neoadjuvant therapy and long-term survival outcomes in breast cancer: a multi-center pooled analysis. Cancer Res. 2020;80(4 Supplement):GS5-GS5-01.
Kantor O, Wong S, Weiss A, Metzger O, Mittendorf EA, King TA. Prognostic significance of residual nodal disease after neoadjuvant endocrine therapy for hormone receptor-positive breast cancer. NPJ Breast Cancer. 2020;6(1):1–6.
Mamounas EP, Anderson SJ, Dignam JJ, Bear HD, Julian TB, Geyer CE, et al. Predictors of locoregional recurrence after neoadjuvant chemotherapy: results from combined analysis of National Surgical Adjuvant Breast and Bowel Project B-18 and B-27. J Clin Oncol. 2012;30(32):3960–6.
Donker M, van Tienhoven G, Straver ME, Meijnen P, van de Velde CJH, Mansel RE, et al. Radiotherapy or surgery of the axilla after a positive sentinel node in breast cancer (EORTC 10981-22023 AMAROS): a randomised, multicentre, open-label, phase 3 non-inferiority trial. Lancet Oncol. 2014;15(12):1303–10.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Human and animal rights and informed consent
This article does not contain any studies with human or animal subjects performed by the authors.
Conflict of interest
Lajos Pusztai has received consulting fees and honoraria from Seagen, Pfizer, Astra Zeneca, Merck, Novartis, Bristol Myers Squibb, Pfizer, Genentech, Eisai, Pieris, Immunomedics, Clovis, Syndax, H3Bio, Radius Health, and Daiichi, and institutional research funding from Seagen, AstraZeneca, Merck, Pfizer and Bristol Myers Squibb. The rest of the authors have no conflicts of interest to disclose.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Breast Cancer
Rights and permissions
About this article
Cite this article
Foldi, J., Rozenblit, M., Park, T.S. et al. Optimal Management for Residual Disease Following Neoadjuvant Systemic Therapy. Curr. Treat. Options in Oncol. 22, 79 (2021). https://doi.org/10.1007/s11864-021-00879-4
Accepted:
Published:
DOI: https://doi.org/10.1007/s11864-021-00879-4