Opinion statement
Prostate cancer is the second leading cause of cancer death in men, and cardiovascular disease is the number one cause of death in patients with prostate cancer. Androgen deprivation therapy, the cornerstone of prostate cancer treatment, has been associated with adverse cardiovascular events. Emerging data supports decreased cardiovascular risk of gonadotropin releasing hormone (GnRH) antagonists compared to agonists. Ongoing clinical trials are assessing the relative safety of different modalities of androgen deprivation therapy. Racial disparities in cardiovascular outcomes in prostate cancer patients are starting to be explored. An intriguing inquiry connects androgen deprivation therapy with reduced risk of COVID-19 infection susceptibility and severity. Recognition of the cardiotoxicity of androgen deprivation therapy and aggressive risk factor modification are crucial for optimal patient care.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Prostate cancer is the second most common cancer overall and the second leading cause of cancer death in men [1]. Androgen deprivation therapy (ADT) is widely used in prostate cancer treatment because excessive androgen receptor activation in prostate cancer cells leads to uncontrolled cell proliferation [2, 3]. ADT reduces testosterone levels to castration levels, disrupting the hypothalamus-pituitary-gonadal axis. ADT can be achieved surgically via bilateral orchiectomy. However, hormone therapy with gonadotropin-releasing hormone (GnRH) agonists and GnRH antagonists is most commonly used.
Within 6 months of diagnosis, 40% of patients with prostate cancer undergo ADT. Almost 50% of patients receive ADT at some point during their illness [4, 5]. ADT is increasingly being used even in the early stages of the disease, along with radiotherapy [6, 7]. When combined with radiotherapy, ADT has been shown to prevent localized prostate cancer progression with high-risk features and improve survival. ADT is also recommended as first-line systemic therapy for metastatic prostate cancer. Exposure to ADT is anticipated to increase with the aging population and earlier prostatic cancer detection.
Despite its efficacy in treating prostate cancer, ADT has been associated with several adverse effects, including, but not limited to, insulin resistance, dyslipidemia, obesity, and osteoporosis [8,9,10]. Notably, the accrued body of evidence from several observational and randomized clinical trials (RCTs) have demonstrated increased risk of cardiovascular (CV) adverse events with ADT. Recent studies and currently ongoing clinical trials seek to determine the safety of GnRH antagonists relative to GnRH agonists from a CV risk perspective.
Cardiovascular disease (CVD) is the leading cause of death in patients with prostate cancer, according to data from the Surveillance, Epidemiology, and End Results (SEER) [11, 12]. Patients with prostate cancer have a higher incidence of CVD than patients without prostate cancer [11]. Death from CVD accounts for 32% (ischemic heart disease 26%, cerebrovascular 6%), while death from prostate cancer stands at number two (20%), followed by death from other causes (14%) [13]. How ADT contributes to CVD in patients with prostate cancer remains an area of active investigation. It is important to remember that CVD and prostate cancer in men have multiple shared risk factors, including smoking, dyslipidemia, obesity, old age, and male gender [14,15,16]. In a cohort study of men with intermediate- or high-risk localized prostate cancer, high Framingham risk scores were found in 65% of patients emphasizing the high degree interaction of common risk factors in these two conditions [17].
This review discusses the CVD toxicities of ADT, emphasizing recent areas of inquiry, including different CVD risk profiles between GnRH agonists versus antagonists, racial disparities in CV outcomes for prostate cancer patients, and the connection between COVID-19 infection susceptibility and ADT.
ADT and risk of cardiovascular disease
The association of ADT with adverse CV outcomes has been a controversial topic due to conflicting results in early published works [18]. Initial sizeable observational cohort studies and a meta-analysis reported no association between ADT and CV morbidity and mortality [19,20,21]. Subsequently, several large observational cohorts and clinical trial meta-analyses have validated the association between ADT and increased CVD mortality and morbidity [22,23,24,25,26,27,28,29,30,31,32,33,34]. For instance, a meta-analysis of eight large observational studies demonstrated that ADT with GnRH agonists was associated with increased risk of the CVD endpoint of nonfatal myocardial infarction (MI), fatal MI, or stroke (RR 1.57) [30]. In a large population-based study of 22,810 newly diagnosed prostate cancer patients, patients receiving a GnRH agonist for at least 1 year were found to have a 20% higher risk of CV events (hazard ratio [HR] 1.2) over a 5-year follow-up period compared with patients who did not receive ADT [25]. In a pooled analysis of three RCTs that included 1372 men, patients with prostate cancer aged ≥ 65 years receiving radiation therapy along with 6 months of ADT had shorter times to fatal MI when compared with those who received radiotherapy alone [31].
ADT was strongly associated with increased risk of CV morbidity and mortality in a subset of prostate cancer patients who had preexisting CVD or one or more risk factors for CVD [23, 26,27,28, 32, 33]. In a retrospective cohort study, among 1378 patients with a history of congestive heart failure or MI treated with radiation, adding ADT was associated with increased all-cause mortality with an adjusted HR of 1.76 [33]. The 5-year all-cause mortality for patients with and without ADT was 22.71% and 11.62%, respectively [33]. In a large observational cohort study involving 26,959 men who received GnRH agonists, there was an increased risk of CV events (HR: 1.21) [28]. This risk was further increased (HR: 1.91) in patients with a history of two or more CV events before initiating therapy with GnRH agonists [28]. In addition to preexisting CVD, age > 75 years and the presence of comorbidities were found to increase susceptibility to ADT-associated CV adverse effects [35]. A detailed appraisal of the evidence for the association of ADT with adverse CV events and possible explanations for inconsistent data in the literature can be found in our recent review [18].
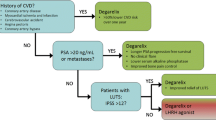
The evidence for increased CV risk in patients with prostate cancer on ADT outweighs the evidence against it, as recognized by national organizations. In 2010, the American Heart Association, American Cancer Society, and American Urological Association published a joint scientific statement to make clinicians and patients aware of the association between ADT and the risk of CV events [36]. In the same year, the US Food and Drug Administration (FDA) issued a statement requiring the addition of safety warning of increased risk of CVD, including heart attack, sudden cardiac death, and stroke on the labeling of GnRH agonist medications. The National Comprehensive Cancer Network (NCCN) guidelines also address ADT-associated CVD risk in prostate cancer patients. In patients with a prior history of significant CVD, referral to a cardiologist before initiating ADT is recommended. NCCN also recommends a multidisciplinary team approach that includes the primary care provider, a geriatrician, and a cardiologist or cardio-oncologist. NCCN further stipulates the assessment of traditional risk factors for CV disease using the ABCDE approach (Awareness and Aspirin, Blood pressure, Cholesterol, and Cigarette smoking, Diet and Diabetes, Exercise) in patients undergoing ADT for prostate cancer [37].
GnRH agonists vs. antagonists: comparison of cardiovascular risk
Although ADT is associated with increased CVD risk, the relative CVD risk between ADT types is now more appreciated. Specifically, evidence of a difference in the CV risk profile of GnRH antagonists (degarelix, abarelix, and relugolix) versus agonists (leuprolide, goserelin, and triptorelin) has been accumulating over the past decade from observational studies, RCTs, and meta-analyses (see Table 1). GnRH agonists are generally preferred by most oncologists, given the long-term experience and the less frequent administration associated with these agents.
Large observational retrospective cohort studies have shown a higher CV risk for GnRH agonists compared with GnRH antagonists. In a retrospective cohort study involving 9785 prostate cancer patients who received ADT, the incidence of CV events was significantly higher in those treated with GnRH agonists rather than antagonists. In the multivariable regression analysis, the risk of adverse CV events was significantly lower in patients treated with GnRH antagonist compared to those treated with GnRH agonists with HR of 0.76 [38]. Similar results were observed in patients without a prior CVD history. In a population-based cohort study conducted in the UK involving 9081 patients, the relative risk of cardiac events was lower with degarelix, a GnRH antagonist, compared with GnRH agonists (HR = 0.39) [39].
Meta-analyses of randomized clinical trials have also found a difference in the CV risk profiles of GnRH agonists versus antagonists [40,41,42]. In a large meta-analysis of pooled data from five phase III RCTs consisting of 1925 patients, degarelix improved prostate-specific antigen (PSA) progression-free survival and overall survival compared to GnRH agonists with HR: 0.71 and HR: 0.47, respectively [41]. The authors inferred that the difference in overall survival was likely due to reduced adverse CV events in the patients who received degarelix. Another large meta-analysis analyzed eight RCTs comprising 2632 men with metastatic prostate cancer who received ADT with either GnRH agonist or antagonist. Their analysis revealed that GnRH antagonist treatment was associated with fewer CV adverse events than GnRH agonists (RR: 0.52) [42]. Patients treated with GnRH antagonists also had lower overall mortality rates than patients who received GnRH agonists.
Other studies of patients treated with GnRH agonists versus antagonists have focused on the risk of CV events in patients with preexisting CVD. Using data from a pooled meta-analysis of 6 RCTs comprising a total of 2328 men, the use of degarelix, a GnRH antagonist, was associated with a 40% reduced risk of major adverse CV events and mortality in patients with preexisting CVD compared to GnRH agonist use [43]. Another meta-analysis compared risk of CV events in GnRH antagonist- and agonist-treated patients using pooled RCT and observational data from four studies. Patients treated with GnRH antagonists had decreased CV risk compared to those treated with GnRH agonists (HR 0.597); the risk reduction was more significant in patients with preexisting CV disease (HR 0.44) [44]. A phase 2 randomized clinical trial compared GnRH agonists with antagonists in 80 men with prostate cancer and preexisting CVD. Within 1 year from initiation of ADT, the incidence of adverse CV events was higher in patients who received GnRH agonists (20%) than those who received GnRH antagonists (3%) [45]. Most recently, a phase 3 trial involving 930 patients with advanced prostate cancer studied the efficacy and CV safety of relugolix, a new oral GnRH antagonist, compared to leuprolide, a GnRH agonist [46]. The incidence of major adverse CV events was 2.9% in the relugolix group and 6.2% in the leuprolide group (HR: 0.46; 95% CI, 0.24 to 0.88), representing a 54% CV risk reduction. For men with a history of preexisting CVD, the difference in the incidence of adverse CV events was more pronounced, 3.6% vs. 17.8% in the GnRH antagonist and GnRH agonist groups, respectively.
GnRH antagonist treatment in patients with prostate cancer is associated with lower CV adverse events than GnRH agonist treatment. Clinicians may find this updated information on CV safety helpful when choosing between the two hormonal ADT options to treat prostate cancer patients, particularly in patients with preexisting CVD.
Abiraterone
Abiraterone is a selective inhibitor of androgen biosynthesis by irreversibly blocking the CYP17 enzyme in the adrenals. Inhibition of the CYP17 enzyme decreases the synthesis of the precursors for androgen and cortisol [47]. When used in combination with ADT, abiraterone was associated with improved survival in men with locally advanced or metastatic prostate cancer compared to ADT alone [48]. However, clinical trials have shown that abiraterone is associated with worsening baseline hypertension and new hypertension diagnosis [49]. The mechanism by which abiraterone causes hypertension is thought to be increased production of mineralocorticoids due to the lack of negative feedback on ACTH production due to decreased cortisol production. To decrease the effect on the adverse effect of mineralocorticoid excess, concomitant administration of abiraterone with low prednisone doses has become the standard. However, ADT combined with abiraterone and prednisone was also associated with an increased incidence of hypertension (20% vs. 0%) compared to ADT alone. In addition, there was also an increased incidence of atrial fibrillation in the abiraterone group atrial fibrillation [50]. A recent meta-analysis revealed that the use of abiraterone acetate significantly increased cardiac toxicity in addition to increased risk of hypertension. The risk of cardiac toxicity was higher during the early period of ADT treatment [51].
Potential mechanisms for CV risk profile differences between GnRH agonists and antagonists
ADT in general is associated with metabolic derangements that aggravate CVD risk factors, such as insulin resistance, diabetes, dyslipidemia, and obesity. However, the relative increase of CV events associated with GnRH agonists likely results from unique mechanisms, as both GnRH antagonists and orchiectomy are associated with fewer adverse CV events than GnRH agonists [22, 52]. The reasons for the increased CV risk associated with GnRH agonists are not well understood. Hypotheses for increased CV risk related to GnRH agonists include testosterone fluctuations, follicle-stimulating hormone (FSH) suppression, and immune system activation.
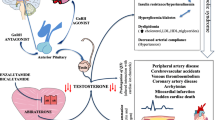
Testosterone fluctuations unique to GnRH agonists may account for the differential risk profiles. In brief, GnRH agonists achieve decreased testosterone levels by exerting negative feedback on the hypothalamus-pituitary axis (Fig. 1). The first administration of GnRH agonists is associated with a significant surge in testosterone levels [53, 54], and each subsequent administration is related to microsurges of testosterone levels [55]. In general, patients treated with GnRH agonists may require weeks or months for a nadir testosterone level to be reached [41]. To counteract the initial testosterone surge, GnRH agonists are frequently administered with androgen receptor blockers (e.g., flutamide, enzalutamide) or androgen synthesis inhibitors (e.g., abiraterone) [56,57,58], which have been independently associated with adverse CV events [59,60,61].
Potential mechanisms of gonadotropin releasing hormone (GnRH) agonist and antagonist cardiotoxicity. GnRH agonist can lead to testosterone microsurges, promote endothelial dysfunction through follicle stimulating hormone (FSH), and directly activate monocytes and T lymphocytes. Together, these actions may promote atherosclerotic plaque formation, disruption, and thrombosis. In contrast, GnRH antagonists do not lead to testosterone microsurges and more rapidly decrease FSH secretion. Both GnRH agonists and antagonists decrease testosterone levels resulting in wide-ranging effects including insulin resistance, adiposity, dyslipidemia, and increased pro-inflammatory mediators.
In contrast, GnRH antagonists, by directly inhibiting GnRH receptors in the anterior pituitary, rapidly suppress testosterone within a few days [62, 63]. Treatment with GnRH antagonists may confer CV benefits by preventing the CV system’s exposure to testosterone surges (Fig. 1), assuming testosterone microsurges are harmful to the CV system. However, both murine and human studies demonstrate that androgens exert beneficial effects on the vasculature with vasodilatory, proliferative, anti-inflammatory, and anti-thrombotic effects [64,65,66,67,68]. Similarly, several animal and human studies have reported that loss of androgens is associated with adverse CV effects. These effects include vascular stiffness, endothelial dysfunction, increased cholesterol content of atherosclerotic lesions, increased plaque vulnerability, prolonged QTc, and increased pro-atherogenic cytokines, fibrinogen, and adiponectin [69,70,71,72,73,74]. Not all studies have reported consistent findings, however, as endothelial injury was reported to be similar in patients with prostate cancer treated with GnRH agonists versus GnRH antagonists [45]. Reconciling these potentially beneficial and deleterious effects of testosterone requires a deeper understanding of factors such as the duration of endothelial exposure to testosterone, the effects of variable serum levels of testosterone on the vasculature, the heterogeneity of endothelial androgen receptors and downstream signaling pathways, and genetic variability (i.e., polymorphisms).
Additionally, FSH levels are significantly more suppressed in patients treated with GnRH antagonists than those who received the GnRH agonists [41, 46]. FSH receptors are found in vascular endothelial cell membranes and promote cell adhesion molecule expression in human tissue culture and animal models (Fig. 1) [75]. FSH has been reported to play a role in cell proliferation, adiposity, and fat distribution [76]. FSH may facilitate endothelial injury and subsequent atheroma formation and progression. Increased levels of FSH have also been associated with prolonged QTc [77]. The clinical significance of these observed changes in the development of CV events in patients treated with GnRH agonists is unknown.
Lastly, GnRH agonists may directly alter the immune system to promote atherosclerotic plaque instability (Fig. 1). In a mouse model, GnRH antagonists were associated with fewer atherosclerotic plaques with instability features than GnRH agonists [78]. In vitro experiments using human peripheral monocytes revealed that GnRH controls the expression of GnRH receptors and interleukin-2 receptor gamma at the messenger RNA level [79, 80]. GnRH agonist signaling could promote T cell differentiation into an inflammatory phenotype in atherosclerotic plaques, thereby facilitating plaque disruption, plaque rupture, and thrombus formation [81, 82].
To delineate the molecular, cellular, and physiologic reasons underpinning the CV risk profile differences between GnRH antagonists and agonists, carefully designed mechanistic studies (both basic and translational) are needed.
Ongoing randomized clinical trials addressing the cardiotoxic effects of GnRH agonists vs. antagonists
In response to the accruing evidence supporting the superior CV safety profile of GnRH antagonists over agonists, three randomized clinical trials are currently ongoing or are in the planning phase (see Table 2). The PRONOUNCE trial (NCT02663908) is a multi-country phase 3 randomized clinical trial in patients with advanced prostate cancer and preexisting atherosclerotic CVD. The CV safety of degarelix, a GnRH antagonist, will be compared prospectively to leuprolide, a GnRH agonist. The primary endpoint is the time from randomization to the first confirmed occurrence of the composite major adverse cardiovascular event (MACE). Although the original plan was to enroll 900 participants, the study enrolled 575 patients before recruitment concluded [83]. NCT04182594 is a smaller phase 2 RCT superiority study comparing the occurrence of CV events in 80 patients with prostate cancer and preexisting CV risk factors receiving degarelix or GnRH agonist in addition to chemotherapy with docetaxel or the newer hormonal agents abiraterone, enzalutamide, or apalutamide [84]. The interventional arm will receive two initial loading doses of 120-mg degarelix for 1 month, followed by 80 mg monthly for eleven additional months. The control arm receives a GnRH agonist at the discretion of the treating urologist/oncologist for 1 year. The primary endpoint will be the time to first composite MACE (see Table 2). PEGASUS is a phase IIIb randomized trial comparing radiation therapy plus long-term adjuvant ADT with GnRH antagonist or GnRH agonist plus flare protection in patients with high-risk localized or locally advanced prostate cancer [85]. The trial’s primary objective is to assess progression-free survival. The principal safety endpoint is the incidence of clinically significant CV events in the subgroup of patients with a prior history of CVD.
When completed, these trials are anticipated to validate the accumulating evidence of the favorable CV safety profile of GnRH agonists compared with GnRH antagonists in patients with preexisting CVD. The studies will also assess whether the superior CV safety of GnRH antagonists persists when treatment is combined with modern regimens of chemotherapy and novel hormonal agents. We propose that a similar high-quality randomized study designed to quantify CV event rates in patients without any prior history of CVD treated with GnRH agonists and antagonists would be an excellent addition to these prospective trials.
Racial disparities in CV outcomes of prostate cancer therapy
Beyond relative CV risks of ADT in prostate cancer therapy, significant racial disparities may adversely impact CV outcomes of patients treated with ADT. African American men are 2.5 times more likely than white men to die from prostate cancer [86]. Between 2008 and 2011, African American men had a mortality rate of 43 per 100,000—significantly higher than men who identified as Caucasian (19.8 per 100,000), Hispanic (17.8 per 100,000), and Asian/Pacific Islanders (9.4 per 100,000) [87]. Studies demonstrate that African American men present at a younger age and with a more aggressive disease at the time of diagnosis and have worse clinical outcomes after radical prostatectomy [88,89,90].
Not only are African Americans disproportionately affected by mortality from prostate cancer, but they also have the highest age-adjusted CV mortality rates. The high mortality from prostate cancer may thus be due in part to higher underlying CV risk and increased adverse CV events associated with ADT. A retrospective cohort involving 7252 men assessed outcomes of low-risk or favorable intermediate-risk prostatic cancer treated with brachytherapy followed by either ADT for a median of 4 months or no ADT [91]. In patients who received ADT, African Americans had significantly increased all-cause mortality (HR 1.77) and non-prostate cancer mortality (HR 1.86) compared to non-African Americans. These racial associations were not observed among men who did not receive ADT.
The underlying causes of these racial disparities are likely multifactorial, complex, and intertwined. Several basic and translational studies have attempted to understand the genetic and molecular basis for the poor outcomes of African American patients with prostate cancer [92,93,94]. However, none of the molecular and genetic factors identified thus far fully explain such a wide gap in mortality from prostate cancer among the different races. Social determinants of health may contribute to the disproportionate deleterious effect of prostate cancer in African American men. These previously described factors include racism, bias, limited access to health care, distrust of the medical community due to prior negative experiences, personal or historical, and low representation in clinical trials.
A study analyzing oncology clinical trials leading to drug approvals between 2008 and 2018 found that although African Americans account for 22% of all cancers in the USA, they constituted just 3.1% of trial participants [95]. Moreover, while one-third of prostate cancer deaths in the USA occur in African Americans, they represented < 5% of participants in sizeable multicenter prostate cancer trials [96]. Improved enrollment of African Americans in clinical trials could be pivotal in defining the best therapies to improve outcomes and reducing adverse CVD events in this community.
ADT in prostate cancer therapy and susceptibility to COVID infection
In the era of the COVID-19 pandemic, cancer is independently associated with adverse outcomes in patients infected with severe acute respiratory syndrome-coronavirus-2 (SARS-COV-2). Patients with a history of cancer and CVD are at significantly higher risk of COVID-19 associated adverse outcomes [97]. Interestingly, ADT-treated prostate cancer patients may experience reduced COVID-19 infections and severity. Using a database from 68 Italian hospitals, researchers identified four COVID-19 infections out of 5273 patients with prostate cancer on ADT [98]. In contrast, 114 COVID-19 infections were identified in 37,161 cancer patients, resulting in a positivity rate of 0.075% (odds ratio 4.05). However, in a northern Italian study investigating only prostate cancer patients, ADT treatment was not associated with decreased COVID-19 infection rates [99]. An American study assessed the clinical course of 58 patients with prostate cancer infected with COVID-19 [100]. After controlling for age, cardiac disease, and pulmonary disease, ADT use was associated with lower hospitalization rates (OR 0.23) and lower supplemental oxygen requirements (OR 0.26). Higher levels of androgens are associated with more severe COVID-19 infections in observational studies of men with androgenic alopecia compared to an age- and race-matched general population [101,102,103].
The biological basis of these clinical observations may be related to androgen regulation of the type II transmembrane serine protease (TMPRSS2). SARS-CoV-2 binds to angiotensin-converting enzyme 2. Then, TMPRSS2 cleavage of the S protein allows the fusion of the virus with the human cellular membrane [104]. The reduction of androgens by ADT may prevent the virus’s entry into cells. Five clinical trials aiming to treat COVID-19 infections with hormonal therapy targeting androgens are currently recruiting or in preparation to recruit (see Table 3).
Conclusion and future directions
ADT-associated cardiotoxicity is linked to increased morbidity and mortality in patients with prostate cancer. Nevertheless, promising data are emerging regarding the improved safety of GnRH antagonists. The risk-benefit ratio between GnRH agonists and antagonists should be carefully considered. It is imperative to identify, monitor, and manage CV risks and complications in prostate cancer patients, especially in older patients with a prior history of significant CVD. The striking racial disparity in prostate cancer mortality among blacks compared to all other races, plus their high mortality from CV disease, warrants prompt attention by the research community. The need for novel strategies to maximize enrollment of these high-risk populations into clinical trials for prostate cancer therapy cannot be overemphasized. More high-quality trials that directly examine adverse CV events as a primary endpoint are needed. Despite the associated cardiotoxicity, ADT intriguingly may confer protection against COVID-19 infection through limiting the virus’ entry into cells.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
American Cancer Society. Key statistics in prostate cancer. 2019 [cited 2019 17 May]; Available from: https://www.cancer.org/cancer/prostate-cancer/about/key-statistics.html.Revised.
Zhou Y, Bolton EC, Jones JO. Androgens and androgen receptor signaling in prostate tumorigenesis. J Mol Endocrinol. 2015;54(1):R15–29.
Packer JR, Maitland NJ. The molecular and cellular origin of human prostate cancer. Biochim Biophys Acta. 2016;1863(6 Pt A):1238–60.
Shahinian VB, Kuo YF, Gilbert SM. Reimbursement policy and androgen-deprivation therapy for prostate cancer. N Engl J Med. 2010;363(19):1822–32.
Scherr D, Swindle PW, Scardino PT. National Comprehensive Cancer Network guidelines for the management of prostate cancer. Urology. 2003;61(2 Suppl 1):14–24.
Shore ND, Antonarakis ES, Cookson MS, Crawford ED, Morgans AK, Albala DM, et al. Optimizing the role of androgen deprivation therapy in advanced prostate cancer: challenges beyond the guidelines. Prostate. 2020;80(6):527–44.
Loblaw DA, Virgo KS, Nam R, Somerfield MR, Ben-Josef E, Mendelson DS, et al. Initial hormonal management of androgen-sensitive metastatic, recurrent, or progressive prostate cancer: 2006 update of an American Society of Clinical Oncology practice guideline. J Clin Oncol. 2007;25(12):1596–605.
Smith MR, Finkelstein JS, McGovern FJ, Zietman AL, Fallon MA, Schoenfeld DA, et al. Changes in body composition during androgen deprivation therapy for prostate cancer. J Clin Endocrinol Metab. 2002;87(2):599–603.
Basaria S, Nguyen T, Rosenson RS, Dobs AS. Effect of methyl testosterone administration on plasma viscosity in postmenopausal women. Clin Endocrinol (Oxf). 2002;57(2):209–14.
Tzortzis V, et al. Adverse effects of androgen deprivation therapy in patients with prostate cancer: focus on metabolic complications. Hormones (Athens). 2017;16(2):115–23.
Sturgeon KM, Deng L, Bluethmann SM, Zhou S, Trifiletti DM, Jiang C, et al. A population-based study of cardiovascular disease mortality risk in US cancer patients. Eur Heart J. 2019;40(48):3889–97.
Leong DP, Fradet V, Shayegan B, Duceppe E, Siemens R, Niazi T, et al. Cardiovascular risk in men with prostate cancer: insights from the RADICAL PC study. J Urol. 2020;203(6):1109–16.
Epstein MM, Edgren G, Rider JR, Mucci LA, Adami HO. Temporal trends in cause of death among Swedish and US men with prostate cancer. J Natl Cancer Inst. 2012;104(17):1335–42.
Platz EA, Till C, Goodman PJ, Parnes HL, Figg WD, Albanes D, et al. Men with low serum cholesterol have a lower risk of high-grade prostate cancer in the placebo arm of the prostate cancer prevention trial. Cancer Epidemiol Biomarkers Prev. 2009;18(11):2807–13.
Discacciati A, Orsini N, Wolk A. Body mass index and incidence of localized and advanced prostate cancer—a dose-response meta-analysis of prospective studies. Ann Oncol. 2012;23(7):1665–71.
Huncharek M, Haddock KS, Reid R, Kupelnick B. Smoking as a risk factor for prostate cancer: a meta-analysis of 24 prospective cohort studies. Am J Public Health. 2010;100(4):693–701.
Davis MK, et al. The prevalence of cardiac risk factors in men with localized prostate cancer undergoing androgen deprivation therapy in British Columbia, Canada. J Oncol. 2015;2015:820403.
• Campbell CM, Zhang KW, Collier A, Linch M, Calaway AC, Ponsky L, et al. Cardiovascular complications of prostate cancer therapy. Current Treatment Options in Cardiovascular Medicine. 2020;22(12):69.This comprehensive review discusses the evidence for cardiotoxicity of prostate cancer therapy beyond androgen deprivation therapy.
Alibhai SM, et al. Impact of androgen deprivation therapy on cardiovascular disease and diabetes. J Clin Oncol. 2009;27(21):3452–8.
Punnen S, Cooperberg MR, Sadetsky N, Carroll PR. Androgen deprivation therapy and cardiovascular risk. J Clin Oncol. 2011;29(26):3510–6.
Nguyen PL, Je Y, Schutz FA, Hoffman KE, Hu JC, Parekh A, et al. Association of androgen deprivation therapy with cardiovascular death in patients with prostate cancer: a meta-analysis of randomized trials. JAMA. 2011;306(21):2359–66.
Keating NL, O'Malley AJ, Smith MR. Diabetes and cardiovascular disease during androgen deprivation therapy for prostate cancer. J Clin Oncol. 2006;24(27):4448–56.
Lester-Coll NH, Goldhaber SZ, Sher DJ, D'Amico AV. Death from high-risk prostate cancer versus cardiovascular mortality with hormonal therapy: a decision analysis. Cancer. 2013;119(10):1808–15.
Keating NL, O'Malley AJ, Freedland SJ, Smith MR. Diabetes and cardiovascular disease during androgen deprivation therapy: observational study of veterans with prostate cancer. J Natl Cancer Inst. 2010;102(1):39–46.
Saigal CS, Gore JL, Krupski TL, Hanley J, Schonlau M, Litwin MS, et al. Androgen deprivation therapy increases cardiovascular morbidity in men with prostate cancer. Cancer. 2007;110(7):1493–500.
Haque R, UlcickasYood M, Xu X, Cassidy-Bushrow AE, Tsai HT, Keating NL, et al. Cardiovascular disease risk and androgen deprivation therapy in patients with localised prostate cancer: a prospective cohort study. Br J Cancer. 2017;117(8):1233–40.
Ziehr DR, Chen MH, Zhang D, Braccioforte MH, Moran BJ, Mahal BA, et al. Association of androgen-deprivation therapy with excess cardiac-specific mortality in men with prostate cancer. BJU Int. 2015;116(3):358–65.
O'Farrell S, Garmo H, Holmberg L, Adolfsson J, Stattin P, van Hemelrijck M. Risk and timing of cardiovascular disease after androgen-deprivation therapy in men with prostate cancer. J Clin Oncol. 2015;33(11):1243–51.
Van Hemelrijck M, et al. Absolute and relative risk of cardiovascular disease in men with prostate cancer: results from the population-based PCBaSe Sweden. J Clin Oncol. 2010;28(21):3448–56.
Bosco C, Bosnyak Z, Malmberg A, Adolfsson J, Keating NL, van Hemelrijck M. Quantifying observational evidence for risk of fatal and nonfatal cardiovascular disease following androgen deprivation therapy for prostate cancer: a meta-analysis. Eur Urol. 2015;68(3):386–96.
D'Amico AV, Denham JW, Crook J, Chen MH, Goldhaber SZ, Lamb DS, et al. Influence of androgen suppression therapy for prostate cancer on the frequency and timing of fatal myocardial infarctions. J Clin Oncol. 2007;25(17):2420–5.
Nanda A, Chen MH, Moran BJ, Braccioforte MH, Dosoretz D, Salenius S, et al. Neoadjuvant hormonal therapy use and the risk of death in men with prostate cancer treated with brachytherapy who have no or at least a single risk factor for coronary artery disease. Eur Urol. 2014;65(1):177–85.
Nguyen PL, Chen MH, Beckman JA, Beard CJ, Martin NE, Choueiri TK, et al. Influence of androgen deprivation therapy on all-cause mortality in men with high-risk prostate cancer and a history of congestive heart failure or myocardial infarction. Int J Radiat Oncol Biol Phys. 2012;82(4):1411–6.
Tsai HK, D'Amico AV, Sadetsky N, Chen MH, Carroll PR. Androgen deprivation therapy for localized prostate cancer and the risk of cardiovascular mortality. J Natl Cancer Inst. 2007;99(20):1516–24.
Morgans AK, Fan KH, Koyama T, Albertsen PC, Goodman M, Hamilton AS, et al. Influence of age on incident diabetes and cardiovascular disease in prostate cancer survivors receiving androgen deprivation therapy. J Urol. 2015;193(4):1226–31.
Levine GN, D'Amico AV, Berger P, Clark PE, Eckel RH, Keating NL, et al. Androgen-deprivation therapy in prostate cancer and cardiovascular risk: a science advisory from the American Heart Association, American Cancer Society, and American Urological Association: endorsed by the American Society for Radiation Oncology. Circulation. 2010;121(6):833–40.
Bhatia N, Santos M, Jones LW, Beckman JA, Penson DF, Morgans AK, et al. Cardiovascular effects of androgen deprivation therapy for the treatment of prostate cancer: ABCDE steps to reduce cardiovascular disease in patients with prostate cancer. Circulation. 2016;133(5):537–41.
• Perrone V, Degli Esposti L, Giacomini E, Veronesi C, Blini V, Oderda M. Cardiovascular risk profile in prostate cancer patients treated with GnRH agonists versus antagonists: an Italian real-world analysis. Ther Clin Risk Manag. 2020;16:393–401.This large Italian study using administrative databases shows GnRH antagonists seem have a better cardiovascular risk profile than GnRH agonists.
• Davey P, Kirby MG. Cardiovascular risk profiles of GnRH agonists and antagonists: real-world analysis from UK general practice. World J Urol. 2020;203:e250–1.This large United Kingdom study from the Optimum Patient Care Research Database found that degarelix conferred significantly lower cardiovascular risk than GnRH agonists.
Sciarra A, Fasulo A, Ciardi A, Petrangeli E, Gentilucci A, Maggi M, et al. A meta-analysis and systematic review of randomized controlled trials with degarelix versus gonadotropin-releasing hormone agonists for advanced prostate cancer. Medicine (Baltimore). 2016;95(27):e3845.
Klotz L, Boccon-Gibod L, Shore ND, Andreou C, Persson BE, Cantor P, et al. The efficacy and safety of degarelix: a 12-month, comparative, randomized, open-label, parallel-group phase III study in patients with prostate cancer. BJU Int. 2008;102(11):1531–8.
Abufaraj M, Iwata T, Kimura S, Haddad A, Al-Ani H, Abusubaih L, Moschini M, Briganti A, Karakiewicz PI, Shariat SF. Differential impact of gonadotropin-releasing hormone antagonist versus agonist on clinical safety and oncologic outcomes on patients with metastatic prostate cancer: a meta-analysis of randomized controlled trials. Eur Urol. 2021;79(1):44–53. https://doi.org/10.1016/j.eururo.2020.06.002
Albertsen PC, Klotz L, Tombal B, Grady J, Olesen TK, Nilsson J. Cardiovascular morbidity associated with gonadotropin releasing hormone agonists and an antagonist. Eur Urol. 2014;65(3):565–73.
Merseburger AS, Sedding D, Huter K. Cardiovascular risk patients under androgen deprivation therapy: lower risk with GnRH antagonists compared to LHRH agonists? Urologe A. 2016;55(2):218–25.
•• Margel D, Peer A, Ber Y, Shavit-Grievink L, Tabachnik T, Sela S, et al. Cardiovascular morbidity in a randomized trial comparing GnRH agonist and GnRH antagonist among patients with advanced prostate cancer and preexisting cardiovascular disease. J Urol. 2019;202(6):1199–208.This randomized trial was the first prospective study to compare cardiovascular outcomes in patients treated with GnRH agonists versus GnRH antagonists.
•• Shore ND, Saad F, Cookson MS, George DJ, Saltzstein DR, Tutrone R, et al. Oral relugolix for androgen-deprivation therapy in advanced prostate cancer. N Engl J Med. 2020;382(23):2187–96.This clinical trial demonstrated the efficacy of the first oral GnRH antagonist compared to GnRH agonist leuprolide to treat prostate cancer and reduce cardiovascular adverse events.
Rehman Y, Rosenberg JE. Abiraterone acetate: oral androgen biosynthesis inhibitor for treatment of castration-resistant prostate cancer. Drug Des Devel Ther. 2012;6:13–8.
James ND, de Bono JS, Spears MR, Clarke NW, Mason MD, Dearnaley DP, et al. Abiraterone for prostate cancer not previously treated with hormone therapy. N Engl J Med. 2017;377(4):338–51.
Ryan CJ, Smith MR, de Bono JS, Molina A, Logothetis CJ, de Souza P, et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med. 2013;368(2):138–48.
Fizazi K, Tran NP, Fein L, Matsubara N, Rodriguez-Antolin A, Alekseev BY, et al. Abiraterone plus prednisone in metastatic, castration-sensitive prostate cancer. N Engl J Med. 2017;377(4):352–60.
Iacovelli R, Ciccarese C, Bria E, Romano M, Fantinel E, Bimbatti D, et al. The cardiovascular toxicity of abiraterone and enzalutamide in prostate cancer. Clin Genitourin Cancer. 2018;16(3):e645–53.
Zhao J, Zhu S, Sun L, Meng F, Zhao L, Zhao Y, et al. Androgen deprivation therapy for prostate cancer is associated with cardiovascular morbidity and mortality: a meta-analysis of population-based observational studies. PLoS One. 2014;9(9):e107516.
van Poppel H, Nilsson S. Testosterone surge: rationale for gonadotropin-releasing hormone blockers? Urology. 2008;71(6):1001–6.
Sharma OP, Weinbauer GF, Behre HM, Nieschlag E. The gonadotropin-releasing hormone (GnRH) agonist-induced initial rise of bioactive LH and testosterone can be blunted in a dose-dependent manner by GnRH antagonist in the non-human primate. Urol Res. 1992;20(5):317–21.
Thompson IM. Flare associated with LHRH-agonist therapy. Rev Urol. 2001;3(Suppl 3):S10–4.
Beer TM, Tombal B. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med. 2014;371(18):1755–6.
Ryan CJ, Molina A, Griffin T. Abiraterone in metastatic prostate cancer. N Engl J Med. 2013;368(15):1458–9.
Trachtenberg J, Gittleman M, Steidle C, Barzell W, Friedel W, Pessis D, et al. A phase 3, multicenter, open label, randomized study of abarelix versus leuprolide plus daily antiandrogen in men with prostate cancer. J Urol. 2002;167(4):1670–4.
Bretagne M, Lebrun-Vignes B, Pariente A, Shaffer CM, Malouf GG, Dureau P, et al. Heart failure and atrial tachyarrhythmia on abiraterone: a pharmacovigilance study. Arch Cardiovasc Dis. 2020;113(1):9–21.
• Salem JE, Yang T, Moslehi JJ, Waintraub X, Gandjbakhch E, Bachelot A, et al. Androgenic effects on ventricular repolarization: a translational study from the international pharmacovigilance database to iPSC-cardiomyocytes. Circulation. 2019;140(13):1070–80.This study identified the arrhythmia effects of enzalutamide through a pharmacovigilance database and demostrated electrophysiologic effects in cardiomyocytes.
Di Nunno V, et al. New hormonal agents in patients with nonmetastatic castration-resistant prostate cancer: meta-analysis of efficacy and safety outcomes. Clin Genitourin Cancer. 2019;17(5):e871–7.
McLeod D, Zinner N, Tomera K, Gleason D, Fotheringham N, Campion M, et al. A phase 3, multicenter, open-label, randomized study of abarelix versus leuprolide acetate in men with prostate cancer. Urology. 2001;58(5):756–61.
Tomera K, et al. The gonadotropin-releasing hormone antagonist abarelix depot versus luteinizing hormone releasing hormone agonists leuprolide or goserelin: initial results of endocrinological and biochemical efficacies in patients with prostate cancer. J Urol. 2001;165(5):1585–9.
Cai JJ, Wen J, Jiang WH, Lin J, Hong Y, Zhu YS. Androgen actions on endothelium functions and cardiovascular diseases. J Geriatr Cardiol. 2016;13(2):183–96.
Bourghardt J, Wilhelmson ASK, Alexanderson C, de Gendt K, Verhoeven G, Krettek A, et al. Androgen receptor-dependent and independent atheroprotection by testosterone in male mice. Endocrinology. 2010;151(11):5428–37.
Hatakeyama H, Nishizawa M, Nakagawa A, Nakano S, Kigoshi T, Uchida K. Testosterone inhibits tumor necrosis factor-alpha-induced vascular cell adhesion molecule-1 expression in human aortic endothelial cells. FEBS Lett. 2002;530(1-3):129–32.
Campelo AE, Cutini PH, Massheimer VL. Testosterone modulates platelet aggregation and endothelial cell growth through nitric oxide pathway. J Endocrinol. 2012;213(1):77–87.
Li S, Li XY, Li J, Deng XL, Li Y, Cong YL. Experimental arterial thrombosis regulated by androgen and its receptor via modulation of platelet activation. Thromb Res. 2007;121(1):127–34.
Dockery F, Bulpitt CJ, Agarwal S, Vernon C, Rajkumar C. Effect of androgen suppression compared with androgen receptor blockade on arterial stiffness in men with prostate cancer. J Androl. 2009;30(4):410–5.
Malkin CJ, Pugh PJ, Jones RD, Jones TH, Channer KS. Testosterone as a protective factor against atherosclerosis—immunomodulation and influence upon plaque development and stability. J Endocrinol. 2003;178(3):373–80.
Gupta D, Lee Chuy K, Yang JC, Bates M, Lombardo M, Steingart RM. Cardiovascular and metabolic effects of androgen-deprivation therapy for prostate cancer. J Oncol Pract. 2018;14(10):580–7.
Salem JE, Waintraub X, Courtillot C, Shaffer CM, Gandjbakhch E, Maupain C, et al. Hypogonadism as a reversible cause of torsades de pointes in men. Circulation. 2018;138(1):110–3.
Gagliano-Juca T, et al. Effects of testosterone replacement on electrocardiographic parameters in men: findings from two randomized trials. J Clin Endocrinol Metab. 2017;102(5):1478–85.
Ziaran S, Goncalves FM, Breza J Sr. Patients with prostate cancer treated by ADT have significantly higher fibrinogenemia than healthy control. World J Urol. 2013;31(2):289–92.
Li X, Chen W, Li P, Wei J, Cheng Y, Liu P, et al. Follicular stimulating hormone accelerates atherogenesis by increasing endothelial VCAM-1 expression. Theranostics. 2017;7(19):4671–88.
Liu XM, Chan HC, Ding GL, Cai J, Song Y, Wang TT, et al. FSH regulates fat accumulation and redistribution in aging through the Galphai/Ca(2+)/CREB pathway. Aging Cell. 2015;14(3):409–20.
Abehsira G, Bachelot A, Badilini F, Koehl L, Lebot M, Favet C, et al. Complex influence of gonadotropins and sex steroid hormones on QT interval duration. J Clin Endocrinol Metab. 2016;101(7):2776–84.
Hopmans SN, Duivenvoorden WCM, Werstuck GH, Klotz L, Pinthus JH. GnRH antagonist associates with less adiposity and reduced characteristics of metabolic syndrome and atherosclerosis compared with orchiectomy and GnRH agonist in a preclinical mouse model. Urol Oncol. 2014;32(8):1126–34.
Chen HF, Jeung EB, Stephenson M, Leung PC. Human peripheral blood mononuclear cells express gonadotropin-releasing hormone (GnRH), GnRH receptor, and interleukin-2 receptor gamma-chain messenger ribonucleic acids that are regulated by GnRH in vitro. J Clin Endocrinol Metab. 1999;84(2):743–50.
Tanriverdi F, Gonzalez-Martinez D, Hu Y, Kelestimur F, Bouloux PMG. GnRH-I and GnRH-II have differential modulatory effects on human peripheral blood mononuclear cell proliferation and interleukin-2 receptor gamma-chain mRNA expression in healthy males. Clin Exp Immunol. 2005;142(1):103–10.
Quyyumi AA. Endothelial function in health and disease: new insights into the genesis of cardiovascular disease. Am J Med. 1998;105(1A):32S–9S.
Dixit VD, Yang H, Udhayakumar V, Sridaran R. Gonadotropin-releasing hormone alters the T helper cytokine balance in the pregnant rat. Biol Reprod. 2003;68(6):2215–21.
ClinicalTrials.gov, A trial comparing cardiovascular safety of degarelix versus leuprolide in patients with advanced prostate cancer and cardiovascular disease (PRONOUNCE).
ClinicalTrials.gov, Cardiovascular events in GnRH agonist vs. antagonist.
ClinicalTrials.gov, Trial comparing irradiation plus long term adjuvant androgen deprivation with GnRH antagonist versus GnRH agonist plus flare protection in patients with very high risk localized or locally advanced prostate cancer (PEGASUS).
Jemal A, Siegel R, Ward E, Murray T, Xu J, Smigal C, et al. Cancer statistics, 2006. CA Cancer J Clin. 2006;56(2):106–30.
Howlader N, Krapcho M, Neyman N, Aminou R, Waldron W, Altekruse SF, et al., editors. SEER cancer statistics review; 2011. p. 1975–2008.
Sundi D, Kryvenko ON, Carter HB, Ross AE, Epstein JI, Schaeffer EM. Pathological examination of radical prostatectomy specimens in men with very low risk disease at biopsy reveals distinct zonal distribution of cancer in black American men. J Urol. 2014;191(1):60–7.
Sundi D, Ross AE, Humphreys EB, Han M, Partin AW, Carter HB, et al. African American men with very low-risk prostate cancer exhibit adverse oncologic outcomes after radical prostatectomy: should active surveillance still be an option for them? J Clin Oncol. 2013;31(24):2991–7.
• Wen W, Luckenbaugh AN, Bayley CE, Penson DF, Shu XO. Racial disparities in mortality for patients with prostate cancer after radical prostatectomy. Cancer. 2020. https://doi.org/10.1002/cncr.33152.This study highlights the survival disparity based on racial identification with adjustments for clinical factors.
Kovtun KA, Chen MH, Braccioforte MH, Moran BJ, D'Amico AV. Race and mortality risk after radiation therapy in men treated with or without androgen-suppression therapy for favorable-risk prostate cancer. Cancer. 2016;122(23):3608–14.
Hashimoto Y, Shiina M, Dasgupta P, Kulkarni P, Kato T, Wong RK, et al. Upregulation of miR-130b contributes to risk of poor prognosis and racial disparity in African-American prostate cancer. Cancer Prev Res (Phila). 2019;12(9):585–98.
Heaphy CM, Joshu CE, Barber JR, Davis C, Zarinshenas R, de Marzo AM, et al. Racial difference in prostate cancer cell telomere lengths in men with higher grade prostate cancer: a clue to the racial disparity in prostate cancer outcomes. Cancer Epidemiol Biomarkers Prev. 2020;29(3):676–80.
Karakas C, Wang C, Deng F, Huang H, Wang D, Lee P. Molecular mechanisms involving prostate cancer racial disparity. Am J Clin Exp Urol. 2017;5(3):34–48.
• Loree JM, Anand S, Dasari A, Unger JM, Gothwal A, Ellis LM, et al. Disparity of race reporting and representation in clinical trials leading to cancer drug approvals from 2008 to 2018. JAMA Oncol. 2019;5:e191870.The lack of diverse racial representation in cancer clinical trials is highlighted in this paper.
Bitting RL, Goodman M, George DJ. Racial disparity in response to prostate cancer systemic therapies. Curr Oncol Rep. 2020;22(9):96.
Ganatra S, Dani SS, Redd R, Rieger-Christ K, Patel R, Parikh R, Asnani A, Bang V, Shreyder K, Brar SS, Singh A, Kazi DS, Guha A, Hayek SS, Barac A, Gunturu KS, Zarwan C, Mosenthal AC, Yunus SA, Kumar A, Patel JM, Patten RD, Venesy DM, Shah SP, Resnic FS, Nohria A, Baron SJ. Outcomes of COVID-19 in patients with a history of cancer and comorbid cardiovascular disease. J Natl Compr Canc Netw. 2020:1–10. https://doi.org/10.6004/jnccn.2020.7658
Montopoli M, Zumerle S, Vettor R, Rugge M, Zorzi M, Catapano CV, et al. Androgen-deprivation therapies for prostate cancer and risk of infection by SARS-CoV-2: a population-based study (N = 4532). Ann Oncol. 2020;31(8):1040–5.
Caffo O, Zagonel V, Baldessari C, Berruti A, Bortolus R, Buti S, et al. On the relationship between androgen-deprivation therapy for prostate cancer and risk of infection by SARS-CoV-2. Ann Oncol. 2020;31(10):1415–6.
Patel VG, Zhong X, Liaw B, Tremblay D, Tsao CK, Galsky MD, et al. Does androgen deprivation therapy protect against severe complications from COVID-19? Ann Oncol. 2020;31(10):1419–20.
Lee J, Yousaf A, Fang W, Kolodney MS. Male balding is a major risk factor for severe COVID-19. J Am Acad Dermatol. 2020;83(5):e353–4.
Wambier CG, Vaño-Galván S, McCoy J, Gomez-Zubiaur A, Herrera S, Hermosa-Gelbard Á, et al. Androgenetic alopecia present in the majority of patients hospitalized with COVID-19: the "Gabrin sign". J Am Acad Dermatol. 2020;83(2):680–2.
Goren A, Vaño-Galván S, Wambier CG, McCoy J, Gomez-Zubiaur A, Moreno-Arrones OM, et al. A preliminary observation: male pattern hair loss among hospitalized COVID-19 patients in Spain - a potential clue to the role of androgens in COVID-19 severity. J Cosmet Dermatol. 2020;19(7):1545–7.
Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–80 e8.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Azariyas A. Challa declares that he has no conflict of interest. Adam Christopher Calaway declares that he has no conflict of interest. Jennifer Cullen declares that she has no conflict of interest. Jorge Garcia declares that he has no conflict of interest. Nihar Desai declares that he has no conflict of interest. Neal L. Weintraub declares that he has no conflict of interest. Anita Deswal declares that she has no conflict of interest. Shelby Kutty declares that he has no conflict of interest. Ajay Vallakati declares that he has no conflict of interest. Daniel Addison declares that he has no conflict of interest. Ragavendra Baliga declares that he has no conflict of interest. Courtney M. Campbell declares that she has no conflict of interest. Avirup Guha declares that he has no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Cardio-oncology
Rights and permissions
About this article
Cite this article
Challa, A.A., Calaway, A.C., Cullen, J. et al. Cardiovascular Toxicities of Androgen Deprivation Therapy. Curr. Treat. Options in Oncol. 22, 47 (2021). https://doi.org/10.1007/s11864-021-00846-z
Accepted:
Published:
DOI: https://doi.org/10.1007/s11864-021-00846-z