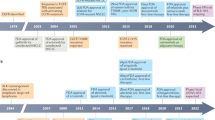
Opinion statement
A decade after the discovery of echinoderm microtubule-associated protein-like 4 (EML4)-anaplastic lymphoma kinase (EML4-ALK) rearrangements in non-small cell lung cancer (NSCLC), several inhibitors have gained regulatory approval, and their sequential use has deferred platinum-based chemotherapy to later lines of therapy. Nevertheless, although most ALK-driven tumors dramatically respond to ALK TKIs , all patients ultimately develop drug-resistant disease. Analysis of post-progression biopsy samples has provided invaluable insight into the mechanisms of resistance, now informing on subsequent therapeutic strategies. In particular, the identification of secondary ALK mutations, which are a common mechanism of resistance to both first-generation and to an even larger extent to second-generation ALK TKIs, may shape a personalized optimal treatment strategy beyond the current first-line choice. Alectinib has now become a preferred treatment option in the first line of therapy, and extrapolation of data obtained from post-progression samples after second-line next-generation ALK TKIs suggests that acquired resistance is likely to be mediated in more than half of patients by ALK resistance mutations. Nevertheless, clinical and preclinical evidence suggests that multiple resistance mechanisms may co-exist at different levels in the same TKI-resistant patient. Newer ALK tyrosine kinase inhibitors (TKIs) overcome some resistance mutations through higher exposure and potency, and generally present greater CNS activity, but are unlikely to overcome resistance mediated through separate oncogenic pathway activations, or epithelial to mesenchymal transition (EMT) and small cell lung cancer (SCLC) transformation. Furthermore, while resistance mutations can be detected through commonly available sequencing methods, the identification of other mechanisms of resistance is much less straightforward in the clinic. We hypothesize that the ALK resistance mutation status will likely be crucially important in the choice of second-line therapy after a second-generation TKI. Emerging clinical data also refines the optimal placing of PD-1- and PD-L1-directed immunotherapy in the treatment sequence.

Similar content being viewed by others
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Duruisseaux M, et al. Overall survival with crizotinib and next-generation ALK inhibitors in ALK-positive non-small-cell lung cancer (IFCT-1302 CLINALK): a French nationwide cohort retrospective study. Oncotarget. 2017;8(13):21903–17.
Kwak EL, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med. 2010;363(18):1693–703.
Soda M, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature. 2007;448(7153):561–6.
Shaw AT, et al. Clinical features and outcome of patients with non-small-cell lung cancer who harbor EML4-ALK. J Clin Oncol. 2009;27(26):4247–53.
Sholl LM, et al. Multi-institutional oncogenic driver mutation analysis in lung adenocarcinoma: the lung cancer mutation consortium experience. J Thorac Oncol. 2015;10(5):768–77.
Takeuchi K, et al. KIF5B-ALK, a novel fusion oncokinase identified by an immunohistochemistry-based diagnostic system for ALK-positive lung cancer. Clin Cancer Res. 2009;15(9):3143–9.
Sasaki T, et al. The biology and treatment of EML4-ALK non-small cell lung cancer. Eur J Cancer. 2010;46(10):1773–80.
Takeuchi K, et al. Multiplex reverse transcription-PCR screening for EML4-ALK fusion transcripts. Clin Cancer Res. 2008;14(20):6618–24.
Choi YL, et al. Identification of novel isoforms of the EML4-ALK transforming gene in non-small cell lung cancer. Cancer Res. 2008;68(13):4971–6.
Koivunen JP, et al. EML4-ALK fusion gene and efficacy of an ALK kinase inhibitor in lung cancer. Clin Cancer Res. 2008;14(13):4275–83.
Hallberg B, Palmer RH. The role of the ALK receptor in cancer biology. Ann Oncol. 2016;27(Suppl 3):iii4–iii15.
Lin JJ, et al. Impact of EML4-ALK variant on resistance mechanisms and clinical outcomes in ALK-positive lung cancer. J Clin Oncol. 2018;36(12):1199–206.
Yoshida T, et al. Differential crizotinib response duration among ALK fusion variants in ALK-positive non-small-cell lung cancer. J Clin Oncol. 2016;34(28):3383–9.
Woo CG, et al. Differential protein stability and clinical responses of EML4-ALK fusion variants to various ALK inhibitors in advanced ALK-rearranged non-small cell lung cancer. Ann Oncol. 2017;28(4):791–7.
Cha YJ, Kim HR, Shim HS. Clinical outcomes in ALK-rearranged lung adenocarcinomas according to ALK fusion variants. J Transl Med. 2016;14(1):296.
Chiarle R, et al. The anaplastic lymphoma kinase in the pathogenesis of cancer. Nat Rev Cancer. 2008;8(1):11–23.
Lindeman NI, et al. Updated molecular testing guideline for the selection of lung cancer patients for treatment with targeted tyrosine kinase inhibitors: guideline from the College of American Pathologists, the International Association for the Study of Lung Cancer, and the Association for Molecular Pathology. J Mol Diagn. 2018;20(2):129–59.
Shaw AT, et al. Lorlatinib in non-small-cell lung cancer with ALK or ROS1 rearrangement: an international, multicentre, open-label, single-arm first-in-man phase 1 trial. Lancet Oncol. 2017;18(12):1590–9.
Roskoski R Jr. Anaplastic lymphoma kinase (ALK) inhibitors in the treatment of ALK-driven lung cancers. Pharmacol Res. 2017;117:343–56.
Zou HY, et al. An orally available small-molecule inhibitor of c-Met, PF-2341066, exhibits cytoreductive antitumor efficacy through antiproliferative and antiangiogenic mechanisms. Cancer Res. 2007;67(9):4408–17.
Blackhall F, et al. Final results of the large-scale multinational trial PROFILE 1005: efficacy and safety of crizotinib in previously treated patients with advanced/metastatic ALK-positive non-small-cell lung cancer. ESMO Open. 2017;2(3):e000219.
Shaw AT, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med. 2013;368(25):2385–94.
Camidge DR, et al. Activity and safety of crizotinib in patients with ALK-positive non-small-cell lung cancer: updated results from a phase 1 study. Lancet Oncol. 2012;13(10):1011–9.
Doebele RC, et al. Mechanisms of resistance to crizotinib in patients with ALK gene rearranged non-small cell lung cancer. Clin Cancer Res. 2012;18(5):1472–82.
Kim DW, et al. Activity and safety of ceritinib in patients with ALK-rearranged non-small-cell lung cancer (ASCEND-1): updated results from the multicentre, open-label, phase 1 trial. Lancet Oncol. 2016;17(4):452–63.
Soria JC, et al. First-line ceritinib versus platinum-based chemotherapy in advanced ALK-rearranged non-small-cell lung cancer (ASCEND-4): a randomised, open-label, phase 3 study. Lancet. 2017;389(10072):917–29.
Cho BC, et al. ASCEND-8: a randomized phase 1 study of ceritinib, 450 mg or 600 mg, taken with a low-fat meal versus 750 mg in fasted state in patients with anaplastic lymphoma kinase (ALK)-rearranged metastatic non-small cell lung cancer (NSCLC). J Thorac Oncol. 2017;12(9):1357–67.
Shaw AT, et al. Alectinib in ALK-positive, crizotinib-resistant, non-small-cell lung cancer: a single-group, multicentre, phase 2 trial. Lancet Oncol. 2016;17(2):234–42.
•• Peters S, et al. Alectinib versus crizotinib in untreated ALK-positive non-small-cell lung cancer. N Engl J Med. 2017;377(9):829–38. Pivotal trial that establishes alectinib as the preferred 1L treatment choice of ALK+ NSCLC.
Hida T, et al. Alectinib versus crizotinib in patients with ALK-positive non-small-cell lung cancer (J-ALEX): an open-label, randomised phase 3 trial. Lancet. 2017;390(10089):29–39.
Kim DW, et al. Brigatinib in patients with crizotinib-refractory anaplastic lymphoma kinase-positive non-small-cell lung cancer: a randomized, multicenter phase II trial. J Clin Oncol. 2017;35(22):2490–8.
Gettinger SN, et al. ORAL33.06 - Brigatinib (AP26113) Efficacy and safety in ALK+ NSCLC: Phase 1/2 Trial Results WCLC. 2015;
Zou HY, et al. PF-06463922, an ALK/ROS1 inhibitor, overcomes resistance to first and second generation ALK inhibitors in preclinical models. Cancer Cell. 2015;28(1):70–81.
Solomon B, et al. OA 05.06 - Phase 2 Study of lorlatinib in patients with advanced ALK+//ROS+/ Non-Small-Cell Lung Cancer (ID 8573) WCLC 2017;
Wakelee HA, et al., MA 07.02 - Response to ensartinib in TKI naïve ALK+ NSCLC patients WCLC. 2017;
Shaw AT, et al. Resensitization to crizotinib by the lorlatinib ALK resistance mutation L1198F. N Engl J Med. 2016;374(1):54–61.
•• Gainor JF, et al. Molecular mechanisms of resistance to first- and second-generation ALK inhibitors in ALK-rearranged lung cancer. Cancer Discov. 2016;6(10):1118–33. Provides invaluable insight into the mechanisms of resistance to crizotinib, alectinib, ceritinib and brigatinib, in the largest series to date.
Shaw AT, et al. Ceritinib in ALK-rearranged non-small-cell lung cancer. N Engl J Med. 2014;370(13):1189–97.
Gainor JF, et al. Progression-free and overall survival in ALK-positive NSCLC patients treated with sequential crizotinib and ceritinib. Clin Cancer Res. 2015;21(12):2745–52.
Cadranel J, et al. Characteristics, treatment patterns, and survival among ALK+ non-small cell lung cancer (NSCLC) patients treated with crizotinib: a chart review study. Lung Cancer. 2016;98:9–14.
Gadgeel SM. Sequencing of ALK inhibitors in ALK+ non-small cell lung cancer. Curr Treat Options in Oncol. 2017;18(6):36.
Yu HA, et al. Acquired resistance of EGFR-mutant lung cancer to a T790M-specific EGFR inhibitor: emergence of a third mutation (C797S) in the EGFR tyrosine kinase domain. JAMA Oncol. 2015;1(7):982–4.
Shaw AT, et al. CT044—efficacy of lorlatinib in patients (pts) with advanced ALK-positive non-small cell lung cancer (NSCLC) and ALK kinase domain mutations. AACR Meeting. 2018;
Govindan R, et al. Genomic landscape of non-small cell lung cancer in smokers and never-smokers. Cell. 2012;150(6):1121–34.
Garassino MC, et al. Durvalumab as third-line or later treatment for advanced non-small-cell lung cancer (ATLANTIC): an open-label, single-arm, phase 2 study. Lancet Oncol. 2018;19(4):521–36.
Felip E, et al. Ceritinib plus nivolumab (NIVO) in patients (pts) with anaplastic lymphoma kinase positive (ALK+) advanced non-small cell lung cancer (NSCLC). J Clin Oncol. 2017;35:15_suppl:2502.
Spigel DR, et al. Phase 1/2 study of the safety and tolerability of nivolumab plus crizotinib for the first-line treatment of ALK translocation-positive advanced non-small cell lung cancer (CheckMate 370). J Thorac Oncol. 2018;13(5):682–88.
Weickhardt AJ, et al. Local ablative therapy of oligoprogressive disease prolongs disease control by tyrosine kinase inhibitors in oncogene-addicted non-small-cell lung cancer. J Thorac Oncol. 2012;7(12):1807–14.
Solomon BJ, et al. Intracranial efficacy of crizotinib versus chemotherapy in patients with advanced ALK-positive non-small-cell lung cancer: results from PROFILE 1014. J Clin Oncol. 2016;34(24):2858–65.
Costa DB, et al. Clinical experience with crizotinib in patients with advanced ALK-rearranged non-small-cell lung cancer and brain metastases. J Clin Oncol. 2015;33(17):1881–8.
Gadgeel S, et al. 1298O_PRAlectinib vs crizotinib in treatment-naïve ALK+ NSCLC: CNS efficacy results from the ALEX study. Ann Oncol. 2017;28(suppl_5):mdx440.057-mdx440.057.
• Kowanetz M, et al., CT076 - IMpower150: efficacy of atezolizumab (atezo) plus bevacizumab (bev) and chemotherapy (chemo) in 1L metastatic nonsquamous NSCLC (mNSCLC) across key subgroups. AACR meeting. 2018; clarifies the optimal role of immune checkpoint inhibitors in ALK+ NSCLC.
Tricker EM, et al. Combined EGFR/MEK inhibition prevents the emergence of resistance in EGFR-mutant lung cancer. Cancer Discov. 2015;5(9):960–71.
Hrustanovic G, et al. RAS-MAPK dependence underlies a rational polytherapy strategy in EML4-ALK-positive lung cancer. Nat Med. 2015;21(9):1038–47.
Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol. 2014;15(3):178–96.
Cha YJ, et al. A case of ALK-rearranged adenocarcinoma with small cell carcinoma-like transformation and resistance to crizotinib. J Thorac Oncol. 2016;11(5):e55–8.
Fujita S, et al. Transformation to SCLC after treatment with the ALK inhibitor alectinib. J Thorac Oncol. 2016;11(6):e67–72.
Levacq D, et al. Histological transformation of ALK rearranged adenocarcinoma into small cell lung cancer: a new mechanism of resistance to ALK inhibitors. Lung Cancer. 2016;102:38–41.
Author information
Authors and Affiliations
Contributions
Solange Peters: conception and design, manuscript writing, and final approval of manuscript. Stefan Zimmermann: conception and design, manuscript writing, and final approval of manuscript. Both authors have contributed equally to the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Lung Cancer
Rights and permissions
About this article
Cite this article
Peters, S., Zimmermann, S. Management of Resistance to First-Line Anaplastic Lymphoma Kinase Tyrosine Kinase Inhibitor Therapy. Curr. Treat. Options in Oncol. 19, 37 (2018). https://doi.org/10.1007/s11864-018-0553-x
Published:
DOI: https://doi.org/10.1007/s11864-018-0553-x