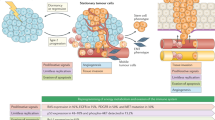
Opinion Statement
Cancer of unknown primary site (CUP) is a clinicopathologic syndrome consisting of many types of cancer and accounting for approximately 3 % of all patients with advanced cancers. This syndrome has frustrated patients and physicians for decades, because a primary site or tissue of origin has not been possible to identify clinically, despite the presence of metastatic tumor. Favorable subsets (approximately 20 % of all CUP) with a presumptive occult primary site have been recognized for several decades based on clinical and standard pathologic features; site-specific therapy in these patients improves their survival compared with the majority of other (approximately 80 %) CUP patients. These other patients, most with adenocarcinomas, have been difficult to treat because the tissue of origin was unknown. Broad-spectrum empiric chemotherapy became the standard approach for these patients in the past 30 years. More recently, new diagnostic technology (evolving immunohistochemistry and emergent gene-expression profiling) has enabled us to establish accurately a tissue of origin in most (90 %+) CUP patients. Gene-expression profiling assays complement standard pathology and for the majority of biopsy specimens accurately identify the primary site or tissue of origin; clinical studies have supported the value of site-directed therapy. When the tissue of origin is in doubt after standard pathologic examination, a gene expression assay is frequently diagnostic, and the outcome of many CUP patients is improved with site-specific therapy. The era of empiric therapy has ended in favor of site-specific therapy, based on the precise diagnosis of the tumor type present in each patient.
Similar content being viewed by others
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Greco FA, Hainsworth JD. Cancer of unknown primary site. In: Devita VT, Lawrence TS, Rosenberg SA, editors. Cancer: principles and practice of oncology. 9th ed. Philadelphia: Lippincott, Williams & Wilkins; 2011. p. 2033–51. This is a comprehensive review covering all the known aspects of the clinicopathologic syndrome of cancer of unknown primary site.
Oien KA. Pathologic evaluation of unknown primary cancer. Semin Oncol. 2009;36:8–37.
Greco FA, Erlander MG. Molecular classification of cancer of unknown primary site. Mol Diagn Ther. 2009;13:367–73.
Greco FA, Spigel DR, Yardley DA, et al. Molecular profiling in unknown primary cancer: accuracy of tissue of origin prediction. Oncol. 2010;15:500–6. This study compared the molecular assay diagnosis to the actual primary site that was found months to years later in patients who initially presented with CUP. These CUP patients who developed latent primaries provide a “gold standard” to test the accuracy of molecular profiling assay.
Kerr SE, Schnabel CA, Sullivan PS, et al. Multisite validation study to determine performance characteristics of a 92-gene molecular cancer classifier. Clin Cancer Res. 2012;18:3953–60.
Monzon FA, Lyons-Weiler M, Burturovic LJ, et al. Multicenter validation of the 1,550-gene expression profile for identification of tumor tissue of origin. J Clin Oncol. 2009;27:2503–8.
Rosenfeld N, Aharonov R, Meiri, et al. MicroRNAs accurately identified cancer tissue origin. Nat Bio Technol. 2008;26:462–9.
Ma XJ, Patel R, Xang, et al. Molecular classification of human cancers using 92-gene real time quantitative polymerase chain reaction assay. Arch Pathol Lab Med. 2006;130:465–73.
Varadhachary GR, Talantov D, Raber MN, et al. Molecular profiling of carcinoma of unknown primary and correlation with clinical evaluation. J Clin Oncol. 2008;26:4442–8.
Horlings HM, van Laar RK, Kerst JM, et al. Gene expression profiling to identify the histogenic origin of metastatic adenocarcinoma of unknown primary. J Clin Oncol. 2008;26:4435–41.
Bridgewater J, van Laar RK, Floore A, et al. Gene expression profiling may improve diagnosis in patients with carcinoma of unknown primary. Br J Cancer. 2008;98:1425–30.
Varadhachary GR, Spector Y, Abbruzzese JL, et al. Prospective gene signature study using micro RNA to identify the tissue of origin in patients with carcinoma of unknown primary. Clin Cancer Res. 2011;17:4063–70.
Greco FA. Cancer of unknown primary site: improved patient management with molecular and immunohistochemical diagnosis. American Society Clinical Oncology Education Book 2013, 175–81.
Greco FA. Cancer of unknown primary site: evolving understanding and management of patients. Clin Adv Hematol Oncol. 2012;10:518–24. This is a relatively brief review and update regarding diagnosis of the tissue of origin and the impact on therapy for patients with unknown primary cancer.
Greco FA, Lennington WJ, Spigel DR, et al. Molecular profiling diagnosis in unknown primary cancer: accuracy and ability to complement standard pathology. J Natl Cancer Inst. 2013;105:782–90. This relatively large study looked at 171 patients with CUP who had molecular profile diagnoses. Three methods were used to determine the accuracy and ability of the molecular assay to complement standard pathology, including accuracy of the diagnosis when compared to the discovery of latent primaries, comparison to single IHC diagnoses and confirmatory IHC staining done following molecular diagnoses. These data establish the accuracy (approximately 80%) of the 92-gene cancer classifier in CUP.
Schwartz AM, Harpaz N. A primary approach to cancer of unknown primary. J Natl Cancer Inst. 2013;105:759–61. This is an accompanying editorial regarding reference 15. The editorial points out the usefulness of molecular diagnosis in CUP and its complementary role to standard pathology.
Weiss LM, Chu P, Schroeder BE, et al. Blinded comparator study of immunohistochemical analysis versus a 92-gene classifier in the diagnosis of the primary site in metastatic tumors. J Mol Diagn. 2013;15:263–9. In this study, IHC was compared to a molecular assay diagnosis and the molecular assay diagnosis compared favorably with IHC, more often diagnosing the tissue of origin correctly, particularly when multiple IHC stains were performed.
Kulkarni A, Pillai R, Ezekiel AM, et al. Comparison of histology to gene expression profiling for the diagnosis of metastatic cancer. Diagn Pathol. 2012;7:1010–113.
Handorf CR, Kulkarni A, Grenert JR, et al. A multicenter study directly comparing the diagnostic accuracy of gene expression profiling and immunohistochemistry for primary site Identification in metastatic tumors. Am J Surg Pathol. 2013;37:1067–75.
Hainsworth JD, Ruben MS, Spigel DR, et al. Molecular gene expression profiling to predict the tissue of origin and direct site-specific therapy in patients with carcinoma of unknown primary site: a prospective trial of the Sarah Cannon Research Institute. J Clin Oncol. 2013;31:217–23. This large, phase II trial provides important information regarding the survival of patients with CUP treated with site-specific therapy based on the molecular assay diagnosis. The median survival of the whole group appeared to be improved compared with a large number of historical control patients treated by the same cooperative group. Perhaps more importantly, patients with molecularly defined tumor types known to be more responsive had a significantly longer survival than those patients with less responsive tumors. It appears that appropriate diagnosis of the tissue of origin can be particularly important for some patients with CUP.
Greco FA, Lennington WJ, et al.: Carcinoma of unknown primary site: outcomes in patients with a colorectal molecular profile treated with site-specific chemotherapy. J Cancer Therapy 2012: 337–43.
Hainsworth JD, Schnabel CA, Erlander MG, et al. A retrospective study of treatment outcomes in patients with carcinoma of unknown primary site and a colorectal cancer molecular profile. Clin Colorectal Cancer. 2012;11:112–8. This retrospective analysis, although subject to patient selection bias, is one of several studies that show a median survival in the CUP colorectal molecularly diagnosed patients comparable to known advanced colorectal cancer following site-specific chemotherapy.
Sorscher SM, Greco FA. Papillary renal carcinoma presenting as a cancer of unknown primary (CUP) and diagnosed through gene expression profiling. Case Rep Oncol. 2012;5:229–32. This represents the first reported case of a CUP patient diagnosed with a molecular assay as renal cell carcinoma. The pathologic and clinical features support this diagnosis and the patient responded to renal targeted therapy.
Hainsworth JD, Spigel DR, Greco FA. Renal cell carcinoma (RCC) presenting as cancer of unknown primary (CUP): diagnosis by molecular tumor profiling (MTP). J Clin Oncol. 2013;31(Suppl; Abstract e 15501). This retrospective review revealed 22 of a total of 444 CUP patients who had a renal gene expression profile detected by the 92-gene RT-PCR assay. The details of these patients revealed they have the clinicopathologic characteristics of metastatic renal cancer and appear to respond similarly to targeted drugs.
Kamposioras K, Pentheroudakis G, Pavlidis N. Exploring the biology of cancer of unknown primary: breakthroughs and drawbacks. Eur J Clin Invest. 2013;43:491–500.
Hainsworth JD, Fizazi K. Treatment for patients with unknown primary cancer and favorable prognostic factors. Sem Oncol. 36:44–52. This review details the favorable CUP subsets and their management.
Pentheroudakis G, Greco FA, Pavlidis N. Molecular assignment of tissue of origin in cancer of unknown primary may not predict response to therapy or outcome: a systematic literature review. Cancer Treat Rev. 2009;35:221–7.
Greco FA, Pavlidis N. Treatment for patients with unknown primary carcinoma and unfavorable prognostic factors. Sem Oncol. 36:65–74. This paper reviews the results of empiric chemotherapy for CUP in the past 15 years.
Pentheroudakis G, Pavlidis N, Fountzilas G, et al. Novel microRNA-based assay demonstrates 92 % agreement with diagnosis based on clinicopathologic and management data in a cohort of patients with carcinoma of unknown primary. Mol Cancer. 2013;12:57. doi:10.1186/1476-4598-12-57. This study showed that the microRNA molecular assay was frequently in agreement with the clinicopathologic diagnoses in CUP.
Varadhachary GR, Raber MN, Matamorsa, et al. Carcinoma of unknown primary with a colon- cancer profile- changing paradigm and emerging definitions. Lancet Oncol. 2008;9:596–608.
Varadhachary GR, Karanth S, Qiaow, et al. Carcinoma of unknown primary with gastrointestinal profile: immunohistochemistry and survival data for this favorable subset. Int J Clin Oncol. 2013 June 28 [E pub ahead of print]. These retrospective data represent a relatively large number of CUP patients who had an immunohistochemical profile and clinical features highly suggestive or diagnostic of a lower gastrointestinal tissue of origin. Most of these patients received colorectal site-specific chemotherapy and their median survival was approximately 30 months. These data support including CUP colorectal profile patients as a favorable CUP subset.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Greco, F.A. Molecular Diagnosis of the Tissue of Origin in Cancer of Unknown Primary Site: Useful in Patient Management. Curr. Treat. Options in Oncol. 14, 634–642 (2013). https://doi.org/10.1007/s11864-013-0257-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11864-013-0257-1