Abstract
Surgical site infections are a common source of post-operative morbidity and contribute significantly to healthcare costs. Patients undergoing emergency laparotomy and/or bowel surgery are particularly at risk. Prophylactic negative pressure wound therapy (NPWT) has been shown to reduce wound infection. However, to date, there has been a lack of consensus around its use for closed laparotomy wounds. We conducted a systematic review of randomised controlled trials comparing the use of prophylactic negative pressure wound therapy with standard dressings for closed laparotomy incisions. The primary outcome was incidence of incisional surgical site infection (SSI) at 30 days post-operatively. Secondary outcomes included superficial and deep SSI, skin dehiscence, fascial dehiscence and length of stay. A total of 2182 publications were identified, of which, following review of titles, abstracts and full texts, five studies met the criteria for inclusion. Across these studies, 467 patients were randomised to NPWT and 464 to standard dressings. Overall SSI rate was 18.6% (n = 87/467) versus 23.9% (n = 111/464) in the NPWT and standard dressing groups, respectively (Odds ratio 0.71, 95% CI 0.52–0.99, p = 0.04*). Deep SSI incidence was the same in both groups (2.6%). Both skin dehiscence and fascial dehiscence were slightly higher in the standard dressing group ((4.2%, n = 11/263 versus 3.1% (n = 8/261) and (0.9% (n = 3/324) versus 0.6% (n = 2/323)), respectively. This study observed that NPWT reduces the overall SSI for closed laparotomy wounds. It supports data recommending the use of prophylactic NPWT dressings, especially in high-risk patients in both emergency and elective circumstances.
Similar content being viewed by others

Introduction
Incisional surgical site infection (SSI) causes significant morbidity to patients and expenditure to healthcare providers [1,2,3]. It can increase hospital stay, promote hernia formation, limit mobilisation and delay commencement of adjuvant chemotherapy and/or interventions [4,5,6,7,8]. In recent years, increased focus and investment have been advocated to reduce SSI rates globally [9], but it remains the most common post-operative morbidity [10, 11]. Recent studies estimate that the incidence of SSI following open abdominal surgery ranges from 15 to 25%, but this is likely to underestimate the true incidence [12,13,14]. Patients undergoing emergency abdominal surgery have significantly higher rates of SSI, especially in the setting of sepsis. Other risk factors include obesity, poor nutritional status, a history of smoking, diabetes and surgery specifically on the bowel [11].
Negative pressure wound therapy (NPWT) is increasingly becoming a well-recognised management option for both open and chronic wounds [15,16,17]. NPWT use in the acute emergency setting following bowel surgery has been proposed to have advantageous benefits in reducing the incidence of SSI. Gomoll et al. first described the successful use of NPWT on closed incisions in a series of orthopaedic trauma cases [18]. Since then, its use has risen exponentially in most surgical specialties [19,20,21,22].
Though there have been several retrospective studies suggesting a large reduction in SSI rates following prophylactic NPWT use [23, 24], there remains a lack of consensus on the evidence from randomised controlled trials (RCT) [25,26,27,28]. Recently, there have been several meta-analyses reporting on NPWT but with considerable inclusion/exclusion limitations.
The aim of this review is to provide the most updated review of current randomised controlled trials producing evidence relating to the prophylactic use of NPWT after laparotomy incisions in general surgical procedures.
Methods
Search strategy
This review was performed according to Cochrane handbook for systematic reviews of interventions [29] and the preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement [30]. A comprehensive search was conducted using PubMed, the Cochrane library database and Clinicaltrials.gov in order to identify RCTs comparing prophylactic negative pressure wound therapy (NWPT) to standard dressings in closed laparotomy wounds published between January 2005 and February 2020. The starting point of 2005 was chosen as this was the first recorded use of prophylactic NPWT. The last date of search was 23 February 2020.
English language texts were searched for using the following search headings: “negative pressure wound therapy” or “npwt” or “pico” or “prevena” and “laparotomy” or “abdominal wound” or “abdominal incision”. All titles were initially evaluated, duplicates were removed. Suitable abstracts were extracted for full-text screening. In addition, each of the eligible publication reference list was also screened for further potential articles.
Inclusion criteria
-
Randomised controlled trials.
-
Comparing NPWT versus standard dressings for closed laparotomy wounds
-
Studies must report on surgical site infection rates for general surgical procedures specifically.
-
English texts only.
Exclusion criteria
-
Did not compare NPWT to standard dressings
-
Non-randomised data
-
Did not report data on laparotomy wound outcomes
-
Gynaecology-only procedures
-
Non-English texts
-
Studies which do not describe key features of study design (such as study type, randomisation, blinding, sample size calculation)
Outcomes
The primary outcome was the incidence of incisional surgical site infection. Secondary outcomes included superficial incisional SSI, deep incisional SSI, skin dehiscence, fascial dehiscence and post-operative length of stay.
Data extraction and statistical analysis
The following data were retrieved from the selected publications: journal, author, year published, country, number of patients per arm, specific treatment applied, control used, emergency/elective ratio, mean/median age, gender breakdown, average BMI, diabetes status and SSI rates. Duplicates were erased and the discrepancies clarified.
Statistical analysis was performed using RevMan statistical software (Ver. 5 Copenhagen, Denmark). Binary outcome data were reported as odds ratios (OR) and 95% confidence interval (95% CI) were estimated using the Mantel-Haenszel method. Heterogeneity was assessed by I-squared statistics, with > 50% being considered as considerable heterogeneity. Statistical significance was attributed to p value < 0.05.
Assessment of risk of Bias
The Cochrane Collaboration’s tool [29] was used to assess risk of bias. Trials were graded as follows: low risk, high risk, and unclear risk. The results of this assessment are depicted in the Appendix Table 4.
Results
Literature search and study characteristics
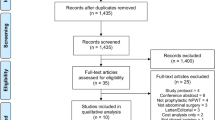
A total of 2182 publications were identified using the aforementioned search criteria. After duplicates were removed, 1996 publications were reviewed. Following the screening of titles and abstracts, ten full texts were assessed for eligibility. Five studies were excluded: one containing a large proportion of gynaecological procedures, one with wounds healed by secondary intention and three which reported on non-randomised data. Five studies were found to meet the predefined inclusion criteria [26,27,28, 31, 32]. Figure 1 depicts the PRISMA flowchart. There was some variance between studies regarding the type of NPWT dressing used, type of control dressing and total days of dressing application. The characteristics of the included RCTs are summarised in Table 1.
Patient characteristics
In total, 467 patients were randomised to NPWT and 464 to control dressings. There were 519 male patients and 412 female patients included in this review. There was no significant difference in average BMI or numbers of diabetics in each arm. Most studies examined elective procedures only. Only 4.7% (n = 44/931) of the included cases were classified as emergencies. These characteristics are illustrated in Table 2.
Outcomes of interest
Overall SSI was higher in the standard dressing group (odds ratio 0.71 (95% CI 0.52–0.99, p = 0.04*, Fig. 2) at 23.9% (n = 111/464) versus 18.6% (n = 87/467)) in the NPWT group.
Only three studies reported on rates of superficial and deep SSIs. Superficial SSIs were higher in the standard dressing group (18.5% (n = 43/232) versus 9.7% (n = 22/227), but there was no difference in deep SSI rates (2.6% in both groups).
Rates of skin dehiscence and fascial dehiscence were lower in the NPWT group, but not statistically different ((4.2%, n = 11/263) versus (3.1% (n = 8/261) and 0.9% (n = 3/324) versus 0.6% (n = 2/323)) for standard and NPWT groups respectively].
Length of stay was only compared in two RCTs. In Murphy et al., the median length of stay was 7 days in both arms, while Flynn et al. observed that the median length of stay in the standard dressing group was 1 day longer (7 versus 8 days). (Table 3 outlines all SSI outcomes).
Discussion
This review of current RCT data observed that the overall SSI rates are significantly reduced with the use of prophylactic NPWT. This supports older meta-analytical data examining the prophylactic NPWT on a variety of closed incisions [33]. However, to date there has been a lack of consensus on the routine use of NPWT following for all laparotomies, not just focusing on emergency cases. A meta-analysis in 2018 noted a decrease in overall SSI rates in NPWT patients [34], but there was considerable heterogeneity in the type of studies included, with one major critique being the inclusion of a RCT which applied NPWT to open rather than closed incisions [35, 36].
In contrast, Kuper et al. did not observe a reduction in SSI (relative risk 0.56, 95% confidence interval 0.30–1.03) and suggested the use of prophylactic NPWT in general and colorectal surgery be “tempered” [35]. However, this review included a study which had a high proportion of patients undergoing gynaecology procedures [25]. This makes the applicability of this review to general surgery questionable. The microbiome of the female reproductive system differs significantly from that of the bowel [37], and as previously stated, resectional bowel surgery carries a significantly higher risk of wound contamination and subsequent SSI. It was for these reasons that data pertaining to gynaecology procedures was excluded from analysis in this study.
SSI encompasses a spectrum of wound issues, ranging from simple cellulitis treated with oral antibiotics to chronic wound issues and/or dehiscence which can be a significant burden to both the patient and the healthcare system. The economic costs associated with SSI are significant. In 2008, individual patient costs in NHS hospitals in Britain ranged from £814 to £6626 depending on the severity of wound infection. The financial impact of SSI on the NHS budget is in excess of £90 million per year, largely due to increased length of stay [38]. The cost of single-use NPWT systems ranges from 150 to 330 euros. Cost-analysis performed by the NICE group suggested that the initial additional cost is offset by a reduction in SSI and its impact to healthcare [39]. However, a recent Cochrane review questioned the true cost-effectiveness of NPWT [40]. Surgical site infections do result in the use of avoidable antibiotic therapy, further intervention for wound management, increased length of stay and have substantial impact on overall recovery and patient well-being [4, 5].
NPWT acts by several mechanisms to improve wound healing. Firstly, it creates a hypoxic environment, which gives rise to increased levels of circulating interleukins (IL-8 and IL-10) and growth factor expression. This stimulates angiogenesis, granulation and extracellular matrix remodelling [41,42,43]. Mechanically, by creating a negative pressure environment, it may act to inhibit seroma formation, thereby lowering bacterial bioburden and promoting wound contraction [44,45,46]. The air-tight seal and suction applied improves wound edge opposition but may also prevent fluids from seeping through the wound onto the patient and the surrounding environment. In light of the COVID-19 pandemic, NPWT may be a valuable tool in reducing spread by forming a cleaner wound area, reducing the number of dressing changes required and potentially reducing length of stay. However, to date, there are no notable studies or recommendations regarding the use of NPWT during the COVID-19 pandemic.
Patients undergoing emergency laparotomy are particularly underrepresented in the RCTs published to date despite having a significantly increased risk of SSI. Forty-three of the 44 emergency cases in this study were included in the study by Flynn et al. However, these patients are described in the study as “sub-acute”. Sub-acute was defined as patients admitted under emergency conditions and requiring surgery on that admission but not immediate surgery [32]. Going forward, it is clear that RCTs must recruit more true emergency cases for analysis, ensuring that study populations are representative of the real world. Patients selected for inclusion should be those that are at increased risk of SSI development. A validated tool to predict SSI risk such that proposed by Ejaz et al. may be beneficial in identifying this cohort of patients [47].
We acknowledge that this study has some limitations. Included patients are from RCT data only, and therefore, intrinsic selection bias is evident. In addition, there is no data on patient reported outcomes, long-term outcomes such as cosmesis, or cost analysis.
The need for further large, multicentre RCTs examining the use of NPWT in laparotomy has been outlined in recent reviews. Such studies would facilitate sub-group analysis of acute versus elective surgery and cost-benefit assessment while providing clarity on appropriate patient selection, duration of treatment and potential limitations [48].
Conclusion
This study observed a significant reduction of overall SSI rates in closed laparotomy wounds treated with prophylactic NPWT. It supports recommendations that the use of NPWT dressings, especially in high-risk patients, in both emergency and elective settings can have considerable benefit to patient recovery.
Availability of data and material
All data previously published and referenced.
Code availability
Not applicable.
References
Pearson A (2009) Historical and changing epidemiology of healthcare-associated infections. J Hosp Infect 73(4):296–304
Konishi T, Watanabe T, Kishimoto J, Nagawa H (2006) Elective colon and rectal surgery differ in risk factors for wound infection: results of prospective surveillance. Ann Surg 244(5):758–763
Zimlichman E, Henderson D, Tamir O, Franz C, Song P, Yamin CK, Keohane C, Denham CR, Bates DW (2013) Health care-associated infections: a meta-analysis of costs and financial impact on the US health care system. JAMA Intern Med 173(22):2039–2046
Smith RL, Bohl JK, McElearney ST, Friel CM, Barclay MM, Sawyer RG, Foley EF (2004) Wound infection after elective colorectal resection. Ann Surg 239(5):599–605 discussion 605-7
Mangram AJ et al (1999) Guideline for prevention of surgical site infection, 1999. Centers for Disease Control and Prevention (CDC) hospital infection control practices advisory committee. Am J Infect Control 27(2):97–132 quiz 133-4; discussion 96
Itatsu K, Yokoyama Y, Sugawara G, Kubota H, Tojima Y, Kurumiya Y, Kono H, Yamamoto H, Ando M, Nagino M (2014) Incidence of and risk factors for incisional hernia after abdominal surgery. Br J Surg 101(11):1439–1447
Tevis SE, Kohlnhofer BM, Stringfield S, Foley EF, Harms BA, Heise CP, Kennedy GD (2013) Postoperative complications in patients with rectal cancer are associated with delays in chemotherapy that lead to worse disease-free and overall survival. Dis Colon Rectum 56(12):1339–1348
de Lissovoy G, Fraeman K, Hutchins V, Murphy D, Song D, Vaughn BB (2009) Surgical site infection: incidence and impact on hospital utilization and treatment costs. Am J Infect Control 37(5):387–397
Berríos-Torres SI et al (2017) Centers for disease control and prevention guideline for the prevention of surgical site infection, 2017. JAMA Surg 152(8):784–791
Mujagic E, Zwimpfer T, Marti WR, Zwahlen M, Hoffmann H, Kindler C, Fux C, Misteli H, Iselin L, Lugli A, Nebiker CA, von Holzen U, Vinzens F, von Strauss M, Reck S, Kraljević M, Widmer AF, Oertli D, Rosenthal R, Weber WP (2014) Evaluating the optimal timing of surgical antimicrobial prophylaxis: study protocol for a randomized controlled trial. Trials 15:188
Taylor GD et al (1995) The effect of surgical wound infection on postoperative hospital stay. Can J Surg 38(2):149–153
Mihaljevic AL et al (2014) Multicenter double-blinded randomized controlled trial of standard abdominal wound edge protection with surgical dressings versus coverage with a sterile circular polyethylene drape for prevention of surgical site infections: a CHIR-net trial (BaFO; NCT01181206). Ann Surg 260(5):730–737 discussion 737-9
Diener MK, Knebel P, Kieser M, Schüler P, Schiergens TS, Atanassov V, Neudecker J, Stein E, Thielemann H, Kunz R, von Frankenberg M, Schernikau U, Bunse J, Jansen-Winkeln B, Partecke LI, Prechtl G, Pochhammer J, Bouchard R, Hodina R, Beckurts KTE, Leißner L, Lemmens HP, Kallinowski F, Thomusch O, Seehofer D, Simon T, Hyhlik-Dürr A, Seiler CM, Hackert T, Reissfelder C, Hennig R, Doerr-Harim C, Klose C, Ulrich A, Büchler MW (2014) Effectiveness of triclosan-coated PDS plus versus uncoated PDS II sutures for prevention of surgical site infection after abdominal wall closure: the randomised controlled PROUD trial. Lancet 384(9938):142–152
Pinkney TD, Calvert M, Bartlett DC, Gheorghe A, Redman V, Dowswell G, Hawkins W, Mak T, Youssef H, Richardson C, Hornby S, Magill L, Haslop R, Wilson S, Morton D, West Midlands Research Collaborative, ROSSINI Trial Investigators (2013) Impact of wound edge protection devices on surgical site infection after laparotomy: multicentre randomised controlled trial (ROSSINI trial). BMJ 347:f4305
Morykwas MJ, Argenta LC (1997) Nonsurgical modalities to enhance healing and care of soft tissue wounds. J South Orthop Assoc 6(4):279–288
Lord AC, Hompes R, Venkatasubramaniam A, Arnold S (2015) Successful management of abdominal wound dehiscence using a vacuum assisted closure system combined with mesh-mediated medial traction. Ann R Coll Surg Engl 97(1):e3–e5
Mukhi AN, Minor S (2014) Management of the open abdomen using combination therapy with ABRA and ABThera systems. Can J Surg 57(5):314–319
Gomoll AH, Lin A, Harris MB (2006) Incisional vacuum-assisted closure therapy. J Orthop Trauma 20(10):705–709
Matatov T, Reddy KN, Doucet LD, Zhao CX, Zhang WW (2013) Experience with a new negative pressure incision management system in prevention of groin wound infection in vascular surgery patients. J Vasc Surg 57(3):791–795
Chadi SA, Kidane B, Britto K, Brackstone M, Ott MC (2014) Incisional negative pressure wound therapy decreases the frequency of postoperative perineal surgical site infections: a cohort study. Dis Colon Rectum 57(8):999–1006
Stannard JP, Volgas DA, McGwin G III, Stewart RL, Obremskey W, Moore T, Anglen JO (2012) Incisional negative pressure wound therapy after high-risk lower extremity fractures. J Orthop Trauma 26(1):37–42
Atkins BZ, Wooten MK, Kistler J, Hurley K, Hughes GC, Wolfe WG (2009) Does negative pressure wound therapy have a role in preventing poststernotomy wound complications? Surg Innov 16(2):140–146
Schurtz E, Differding J, Jacobson E, Maki C, Ahmeti M (2018) Evaluation of negative pressure wound therapy to closed laparotomy incisions in acute care surgery. Am J Surg 215(1):113–115
Zaidi A, El-Masry S (2017) Closed-incision negative-pressure therapy in high-risk general surgery patients following laparotomy: a retrospective study. Color Dis 19(3):283–287
O'Leary DP et al (2017) Prophylactic negative pressure dressing use in closed laparotomy wounds following abdominal operations: a randomized, controlled, open-label trial: the P.I.C.O. trial. Ann Surg 265(6):1082–1086
Murphy PB et al (2018) Negative pressure wound therapy use to decrease surgical nosocomial events in colorectal resections (NEPTUNE): a randomized controlled trial. Ann Surg
Shen P, Blackham AU, Lewis S, Clark CJ, Howerton R, Mogal HD, Dodson RM, Russell GB, Levine EA (2017) Phase II randomized trial of negative-pressure wound therapy to decrease surgical site infection in patients undergoing laparotomy for gastrointestinal, pancreatic, and peritoneal surface malignancies. J Am Coll Surg 224(4):726–737
Javed AA, Teinor J, Wright M, Ding D, Burkhart RA, Hundt J, Cameron JL, Makary MA, He J, Eckhauser FE, Wolfgang CL, Weiss MJ (2019) Negative pressure wound therapy for surgical-site infections: a randomized trial. Ann Surg 269(6):1034–1040
Higgins JP et al (2011) The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. Bmj 343:d5928
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, Clarke M, Devereaux PJ, Kleijnen J, Moher D (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol 62(10):e1–e34
Li PY, Yang D, Liu D, Sun SJ, Zhang LY (2017) Reducing surgical site infection with negative-pressure wound therapy after open abdominal surgery: a prospective randomized controlled study. Scand J Surg 106(3):189–195
Flynn J et al (2019) Negative pressure dressings (PICO(TM)) on laparotomy wounds do not reduce risk of surgical site infection. Surg Infect
Hyldig N, Birke-Sorensen H, Kruse M, Vinter C, Joergensen JS, Sorensen JA, Mogensen O, Lamont RF, Bille C (2016) Meta-analysis of negative-pressure wound therapy for closed surgical incisions. Br J Surg 103(5):477–486
Sahebally SM, McKevitt K, Stephens I, Fitzpatrick F, Deasy J, Burke JP, McNamara D (2018) Negative pressure wound therapy for closed laparotomy incisions in general and colorectal surgery: a systematic review and meta-analysis. JAMA Surg 153(11):e183467
Kuper TM, Murphy PB, Kaur B, Ott MC (2020) Prophylactic negative pressure wound therapy for closed laparotomy incisions: a meta-analysis of randomized controlled trials. Ann Surg 271(1):67–74
Lozano-Balderas G, Ruiz-Velasco-Santacruz A, Diaz-Elizondo JA, Gomez-Navarro JA, Flores-Villalba E (2017) Surgical site infection rate drops to 0% using a vacuum-assisted closure in contaminated/dirty infected laparotomy wounds. Am Surg 83(5):512–514
Chase D, Goulder A, Zenhausern F, Monk B, Herbst-Kralovetz M (2015) The vaginal and gastrointestinal microbiomes in gynecologic cancers: a review of applications in etiology, symptoms and treatment. Gynecol Oncol 138(1):190–200
Payne C, Edwards D (2014) Application of the single use negative pressure wound therapy device (PICO) on a heterogeneous group of surgical and traumatic wounds. Eplasty 14:e20
Excellence, N.I.f.H.a. PICO Negative pressure wound dressings for closed surgical incisions. 2019; Available from: https://www.nice.org.uk/process/pmg6/resources/how-nice-clinical-guidelines-are-developed-an-overview-for-stakeholders-the-public-and-the-nhs-2549708893/chapter/nice-clinical-guidelines. Accessed April 2020
Webster J et al (2019) Negative pressure wound therapy for surgical wounds healing by primary closure. Cochrane Database Syst Rev 3:Cd009261
Glass GE, Murphy GF, Esmaeili A, Lai LM, Nanchahal J (2014) Systematic review of molecular mechanism of action of negative-pressure wound therapy. Br J Surg 101(13):1627–1636
Huang C, Leavitt T, Bayer LR, Orgill DP (2014) Effect of negative pressure wound therapy on wound healing. Curr Probl Surg 51(7):301–331
Ma Z et al (2016) Negative pressure wound therapy promotes vessel destabilization and maturation at various stages of wound healing and thus influences wound prognosis. Exp Ther Med 11(4):1307–1317
Weed T, Ratliff C, Drake DB (2004) Quantifying bacterial bioburden during negative pressure wound therapy: does the wound VAC enhance bacterial clearance? Ann Plast Surg 52(3):276–279 discussion 279-80
Banwell P, Withey S, Holten I (1998) The use of negative pressure to promote healing. Br J Plast Surg 51(1):79
López-Cano M, Armengol-Carrasco M (2013) Use of vacuum-assisted closure in open incisional hernia repair: a novel approach to prevent seroma formation. Hernia 17(1):129–131
Ejaz A, Schmidt C, Johnston FM, Frank SM, Pawlik TM (2017) Risk factors and prediction model for inpatient surgical site infection after major abdominal surgery. J Surg Res 217:153–159
Fowler AL, Barry MK (2019) Closed incision negative pressure therapy: results of recent trials and recommendations for clinical practice. Surgeon
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
All authors declare that they have no conflict of interest.
Ethics approval
Review of published data. Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
Rights and permissions
About this article
Cite this article
Boland, P.A., Kelly, M.E., Donlon, N.E. et al. Prophylactic negative pressure wound therapy for closed laparotomy wounds: a systematic review and meta-analysis of randomised controlled trials. Ir J Med Sci 190, 261–267 (2021). https://doi.org/10.1007/s11845-020-02283-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11845-020-02283-7