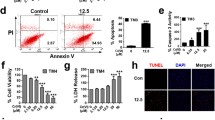
Abstract
Background
Cadmium (Cd) is an environmental and industrial pollutant that induces a broad spectrum of toxicological effects, influences a variety of human organs, and is associated with poor semen quality and male infertility. Increasing evidence demonstrates that Cd induces testicular germ cell apoptosis in rodent animals. However, the specific effect of Cd exposure on autophagy in germ cells is poorly understood.
Methods
We investigate the role of high-mobility group box 1 protein (HMGB1), a ubiquitous nuclear protein, on Cd-evoked autophagy in a mouse spermatocyte cell line (GC-2spd).
Results
Our data have shown that autophagy was significantly elevated in GC-2spd cells exposed to Cd. Furthermore, there was a reduction in rapamycin (RAP)-mediated apoptosis. In addition, Cd exposure reduced cell viability, which is an effect that could be significantly inhibited by RAP treatment. These results indicate that autophagy appears to serve a positive function in reducing Cd-induced cytotoxicity. In addition, HMGB1 increased coincident with the processing of LC3-I to LC3-II. Thus, the upregulation of HMGB1 increases LC3-II levels.
Conclusions
Our data suggest that HMGB1-induced autophagy appears to act as a defense/survival mechanism against Cd cytotoxicity in GC-2spd cells.




Similar content being viewed by others
References
Honda R, Swaddiwudhipong W, Nishijo M, Mahasakpan P, Teeyakasem W, Ruangyuttikarn W, Satarug S, Padungtod C, Nakagawa H (2010) Cadmium induced renal dysfunction among residents of rice farming area downstream from a zinc-mineralized belt in Thailand. Toxicol Lett 198(1):26–32. doi:10.1016/j.toxlet.2010.04.023
Pant N, Upadhyay G, Pandey S, Mathur N, Saxena DK, Srivastava SP (2003) Lead and cadmium concentration in the seminal plasma of men in the general population: correlation with sperm quality. Reprod Toxicol 17(4):447–450
Monsefi M, Alaee S, Moradshahi A, Rohani L (2010) Cadmium-induced infertility in male mice. Environ Toxicol 25(1):94–102. doi:10.1002/tox.20468
Wu HM, Lin-Tan DT, Wang ML, Huang HY, Wang HS, Soong YK, Lin JL (2008) Cadmium level in seminal plasma may affect the pregnancy rate for patients undergoing infertility evaluation and treatment. Reprod Toxicol 25(4):481–484. doi:10.1016/j.reprotox.2008.04.005
Xu LC, Wang SY, Yang XF, Wang XR (2001) Effects of cadmium on rat sperm motility evaluated with computer assisted sperm analysis. Biomed Environ Sci 14(4):312–317
Waalkes MP (2003) Cadmium carcinogenesis. Mutat Res 533(1–2):107–120
Prozialeck WC, Edwards JR (2012) Mechanisms of cadmium-induced proximal tubule injury: new insights with implications for biomonitoring and therapeutic interventions. J Pharmacol Exp Ther 343(1):2–12. doi:10.1124/jpet.110.166769
Kim J, Soh J (2009) Cadmium-induced apoptosis is mediated by the translocation of AIF to the nucleus in rat testes. Toxicol Lett 188(1):45–51. doi:10.1016/j.toxlet.2009.03.006
Ozawa N, Goda N, Makino N, Yamaguchi T, Yoshimura Y, Suematsu M (2002) Leydig cell-derived heme oxygenase-1 regulates apoptosis of premeiotic germ cells in response to stress. J Clin Investig 109(4):457–467. doi:10.1172/JCI13190
Esakky P, Hansen DA, Drury AM, Moley KH (2014) Modulation of cell cycle progression in the spermatocyte cell line [GC-2spd(ts) Cell-Line] by cigarette smoke condensate (CSC) via arylhydrocarbon receptor-nuclear factor erythroid 2-related factor 2 (Ahr-Nrf2) pathway. Biol Reprod 90(1):9. doi:10.1095/biolreprod.113.113225
Yang X, Zhong X, Tanyi JL, Shen J, Xu C, Gao P, Zheng TM, DeMichele A, Zhang L (2013) mir-30d Regulates multiple genes in the autophagy pathway and impairs autophagy process in human cancer cells. Biochem Biophys Res Commun 431(3):617–622. doi:10.1016/j.bbrc.2012.12.083
Mizushima N, Levine B, Cuervo AM, Klionsky DJ (2008) Autophagy fights disease through cellular self-digestion. Nature 451(7182):1069–1075. doi:10.1038/nature06639
Boya P, Gonzalez-Polo RA, Casares N, Perfettini JL, Dessen P, Larochette N, Metivier D, Meley D, Souquere S, Yoshimori T, Pierron G, Codogno P, Kroemer G (2005) Inhibition of macroautophagy triggers apoptosis. Mol Cell Biol 25(3):1025–1040. doi:10.1128/MCB.25.3.1025-1040.2005
Lum JJ, Bauer DE, Kong M, Harris MH, Li C, Lindsten T, Thompson CB (2005) Growth factor regulation of autophagy and cell survival in the absence of apoptosis. Cell 120(2):237–248. doi:10.1016/j.cell.2004.11.046
Shimizu S, Kanaseki T, Mizushima N, Mizuta T, Arakawa-Kobayashi S, Thompson CB, Tsujimoto Y (2004) Role of Bcl-2 family proteins in a non-apoptotic programmed cell death dependent on autophagy genes. Nat Cell Biol 6(12):1221–1228. doi:10.1038/ncb1192
Yu L, Alva A, Su H, Dutt P, Freundt E, Welsh S, Baehrecke EH, Lenardo MJ (2004) Regulation of an ATG7-beclin 1 program of autophagic cell death by caspase-8. Science 304(5676):1500–1502. doi:10.1126/science.1096645
Gozuacik D, Kimchi A (2007) Autophagy and cell death. Curr Top Dev Biol 78:217–245. doi:10.1016/S0070-2153(06)78006-1
Chen Y, McMillan-Ward E, Kong J, Israels SJ, Gibson SB (2008) Oxidative stress induces autophagic cell death independent of apoptosis in transformed and cancer cells. Cell Death Differ 15(1):171–182. doi:10.1038/sj.cdd.4402233
Tang D, Kang R, Zeh HJ 3rd, Lotze MT (2010) High-mobility group box 1 and cancer. Biochem Biophys Acta 1799(1–2):131–140. doi:10.1016/j.bbagrm.2009.11.014
Tang D, Kang R, Livesey KM, Cheh CW, Farkas A, Loughran P, Hoppe G, Bianchi ME, Tracey KJ, Zeh HJ 3rd, Lotze MT (2010) Endogenous HMGB1 regulates autophagy. J Cell Biol 190(5):881–892. doi:10.1083/jcb.200911078
Tang D, Kang R, Cheh CW, Livesey KM, Liang X, Schapiro NE, Benschop R, Sparvero LJ, Amoscato AA, Tracey KJ, Zeh HJ, Lotze MT (2010) HMGB1 release and redox regulates autophagy and apoptosis in cancer cells. Oncogene 29(38):5299–5310. doi:10.1038/onc.2010.261
Tang D, Kang R, Livesey KM, Zeh HJ 3rd, Lotze MT (2011) High mobility group box 1 (HMGB1) activates an autophagic response to oxidative stress. Antioxid Redox Signal 15(8):2185–2195. doi:10.1089/ars.2010.3666
Zhang Q, Kang R, Zeh HJ 3rd, Lotze MT, Tang D (2013) DAMPs and autophagy: cellular adaptation to injury and unscheduled cell death. Autophagy 9(4):451–458. doi:10.4161/auto.23691
Sun X, Tang D (2014) HMGB1-dependent and -independent autophagy. Autophagy 10(10):1873–1876. doi:10.4161/auto.32184
Jarup L, Akesson A (2009) Current status of cadmium as an environmental health problem. Toxicol Appl Pharmacol 238(3):201–208. doi:10.1016/j.taap.2009.04.020
Glahn F, Schmidt-Heck W, Zellmer S, Guthke R, Wiese J, Golka K, Hergenroder R, Degen GH, Lehmann T, Hermes M, Schormann W, Brulport M, Bauer A, Bedawy E, Gebhardt R, Hengstler JG, Foth H (2008) Cadmium, cobalt and lead cause stress response, cell cycle deregulation and increased steroid as well as xenobiotic metabolism in primary normal human bronchial epithelial cells which is coordinated by at least nine transcription factors. Arch Toxicol 82(8):513–524. doi:10.1007/s00204-008-0331-9
Henson MC, Chedrese PJ (2004) Endocrine disruption by cadmium, a common environmental toxicant with paradoxical effects on reproduction. Exp Biol Med 229(5):383–392
Terada N, Ohno N, Yamakawa H, Ohara O, Liao X, Baba T, Ohno S (2005) Immunohistochemical study of a membrane skeletal molecule, protein 4.1G, in mouse seminiferous tubules. Histochem Cell Biol 124(3–4):303–311. doi:10.1007/s00418-005-0031-y
Zhou T, Jia X, Chapin RE, Maronpot RR, Harris MW, Liu J, Waalkes MP, Eddy EM (2004) Cadmium at a non-toxic dose alters gene expression in mouse testes. Toxicol Lett 154(3):191–200. doi:10.1016/j.toxlet.2004.07.015
Yu X, Hong S, Faustman EM (2008) Cadmium-induced activation of stress signaling pathways, disruption of ubiquitin-dependent protein degradation and apoptosis in primary rat Sertoli cell-gonocyte cocultures. ToxicolSci 104(2):385–396. doi:10.1093/toxsci/kfn087
Benoff S, Jacob A, Hurley IR (2000) Male infertility and environmental exposure to lead and cadmium. Human Reprod Update 6(2):107–121
Kitanaka C, Kuchino Y (1999) Caspase-independent programmed cell death with necrotic morphology. Cell Death Differ 6(6):508–515. doi:10.1038/sj.cdd.4400526
Komatsu M, Waguri S, Chiba T, Murata S, Iwata J, Tanida I, Ueno T, Koike M, Uchiyama Y, Kominami E, Tanaka K (2006) Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature 441(7095):880–884. doi:10.1038/nature04723
Klionsky DJ (2007) Autophagy: from phenomenology to molecular understanding in less than a decade. Nat Rev Mol Cell Biol 8(11):931–937. doi:10.1038/nrm2245
Templeton DM, Liu Y (2010) Multiple roles of cadmium in cell death and survival. Chem Biol Interact 188(2):267–275. doi:10.1016/j.cbi.2010.03.040
Wang SH, Shih YL, Ko WC, Wei YH, Shih CM (2008) Cadmium-induced autophagy and apoptosis are mediated by a calcium signaling pathway. Cell Mol Life Sci 65(22):3640–3652. doi:10.1007/s00018-008-8383-9
Son YO, Wang X, Hitron JA, Zhang Z, Cheng S, Budhraja A, Ding S, Lee JC, Shi X (2011) Cadmium induces autophagy through ROS-dependent activation of the LKB1-AMPK signaling in skin epidermal cells. Toxicol Appl Pharmacol 255(3):287–296. doi:10.1016/j.taap.2011.06.024
Wang Q, Zhu J, Zhang K, Jiang C, Wang Y, Yuan Y, Bian J, Liu X, Gu J, Liu Z (2013) Induction of cytoprotective autophagy in PC-12 cells by cadmium. Biochem Biophys Res Commun 438(1):186–192. doi:10.1016/j.bbrc.2013.07.050
Chargui A, Zekri S, Jacquillet G, Rubera I, Ilie M, Belaid A, Duranton C, Tauc M, Hofman P, Poujeol P, El May MV, Mograbi B (2011) Cadmium-induced autophagy in rat kidney: an early biomarker of subtoxic exposure. Toxicol Sci 121(1):31–42. doi:10.1093/toxsci/kfr031
Lim SC, Hahm KS, Lee SH, Oh SH (2010) Autophagy involvement in cadmium resistance through induction of multidrug resistance-associated protein and counterbalance of endoplasmic reticulum stress WI38 lung epithelial fibroblast cells. Toxicology 276(1):18–26. doi:10.1016/j.tox.2010.06.010
Dong Z, Wang L, Xu J, Li Y, Zhang Y, Zhang S, Miao J (2009) Promotion of autophagy and inhibition of apoptosis by low concentrations of cadmium in vascular endothelial cells. Toxicol Vitro 23(1):105–110. doi:10.1016/j.tiv.2008.11.003
Han W, Sun J, Feng L, Wang K, Li D, Pan Q, Chen Y, Jin W, Wang X, Pan H, Jin H (2011) Autophagy inhibition enhances daunorubicin-induced apoptosis in K562 cells. PLoS One 6(12):e28491. doi:10.1371/journal.pone.0028491
Cheng Y, Qiu F, Ye YC, Guo ZM, Tashiro S, Onodera S, Ikejima T (2009) Autophagy inhibits reactive oxygen species-mediated apoptosis via activating p38-nuclear factor-kappa B survival pathways in oridonin-treated murine fibrosarcoma L929 cells. FEBS J 276(5):1291–1306. doi:10.1111/j.1742-4658.2008.06864.x
Silva-Fernandes A, Duarte-Silva S, Neves-Carvalho A, Amorim M, Soares-Cunha C, Oliveira P, Thirstrup K, Teixeira-Castro A, Maciel P (2014) Chronic treatment with 17-DMAG improves balance and coordination in a new mouse model of Machado-Joseph disease. Neurotherapeutics 11(2):433–449. doi:10.1007/s13311-013-0255-9
Lotze MT, Tracey KJ (2005) High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nat Rev Immunol 5(4):331–342. doi:10.1038/nri1594
Huang J, Ni J, Liu K, Yu Y, Xie M, Kang R, Vernon P, Cao L, Tang D (2012) HMGB1 promotes drug resistance in osteosarcoma. Can Res 72(1):230–238. doi:10.1158/0008-5472.CAN-11-2001
Livesey KM, Kang R, Vernon P, Buchser W, Loughran P, Watkins SC, Zhang L, Manfredi JJ, Zeh HJ 3rd, Li L, Lotze MT, Tang D (2012) p53/HMGB1 complexes regulate autophagy and apoptosis. Can Res 72(8):1996–2005. doi:10.1158/0008-5472.CAN-11-2291
Song JX, Lu JH, Liu LF, Chen LL, Durairajan SS, Yue Z, Zhang HQ, Li M (2014) HMGB1 is involved in autophagy inhibition caused by SNCA/alpha-synuclein overexpression: a process modulated by the natural autophagy inducer corynoxine B. Autophagy 10(1):144–154. doi:10.4161/auto.26751
Liu K, Huang J, Xie M, Yu Y, Zhu S, Kang R, Cao L, Tang D, Duan X (2014) MIR34A regulates autophagy and apoptosis by targeting HMGB1 in the retinoblastoma cell. Autophagy 10(3):442–452. doi:10.4161/auto.27418
Li X, Wang S, Chen Y, Liu G, Yang X (2014) miR-22 targets the 3′ UTR of HMGB1 and inhibits the HMGB1-associated autophagy in osteosarcoma cells during chemotherapy. Tumour Biol 35(6):6021–6028. doi:10.1007/s13277-014-1797-0
Huebener P, Gwak GY, Pradere JP, Quinzii CM, Friedman R, Lin CS, Trent CM, Mederacke I, Zhao E, Dapito DH, Lin Y, Goldberg IJ, Czaja MJ, Schwabe RF (2014) High-mobility group box 1 is dispensable for autophagy, mitochondrial quality control, and organ function in vivo. Cell Metab 19(3):539–547. doi:10.1016/j.cmet.2014.01.014
Acknowledgements
This study was funded by The National Natural Science Foundation of China (31171229 and U1132005), The Natural Science Foundation of Guangdong Province (2014A030312012); International Cooperation Project of Science and Technology Planning Project of Guangdong Province (2013B51000087); Science and Information Technology of Guangzhou Key Project (201508020258, 201400000003-4 and 201400000004-4).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing financial interests.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Ou, Z., Chen, Y., Niu, X. et al. High-mobility group box 1 regulates cytoprotective autophagy in a mouse spermatocyte cell line (GC-2spd) exposed to cadmium. Ir J Med Sci 186, 1041–1050 (2017). https://doi.org/10.1007/s11845-017-1595-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11845-017-1595-y