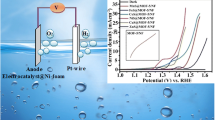
Abstract
Development of facile, low-cost, and highly efficient oxygen evolution reaction (OER) catalysts with excellent oxygen evolution activity and stability has been a major challenge in recent years. In this work, efficient TMSe@MOF-5 catalysts were synthesized by in situ incorporation of presynthesized transition-metal selenide nanoparticles (MnSe, FeSe, CoSe, NiSe, CuSe, and ZnSe) into MOF-5 at room temperature. All the synthesized composites were successfully characterized by powder x-ray diffraction analysis, Fourier-transform infrared spectroscopy, Raman spectroscopy, scanning electron microscopy, energy-dispersive x-ray spectroscopy, elemental mapping, and ultraviolet–visible spectrophotometry. Among various TMSe@MOF-5/NF working electrodes, MnSe@MOF-5/NF showed higher OER catalytic activity, requiring an overpotential of only 170 mV to achieve a current density of 10 mA cm−2 in alkaline medium with a Tafel slope of 61 mV dec−1, which is superior to many other reported OER catalysts including state-of-the-art RuO2 (ƞ10 = 290 mV). It is believed that the higher OER activity of MnSe@MOF-5/NF is due to the formation of a heterojunction from Ni of the nickel foam to the MnSe@MOF-5 at the surface of the working electrode.




Similar content being viewed by others
References
S. Cao, Z. Wu, B. Fu, H. Yu, and L. Piao, Catal. Today 330, 246 (2019).
N. Fajrina and M. Tahir, Int. J. Hydrogen Energy 44, 540 (2019).
N.M. Gupta, Renew. Sust. Energy Rev. 71, 585 (2016).
P.K. Dubey, P. Tripathi, R.S. Tiwari, A.S.K. Sinha, and O.N. Srivastava, Int. J. Hydrogen Energy 39, 16282 (2014).
T. Degnan, Focus Catal. 2015, 1 (2015).
Z. Wang, J. Li, X. Tian, X. Wang, Y. Yu, K.A. Owusu, L. He, L. Mai, and A.C.S. Appl, Mater. Interfaces 8, 19386 (2016).
X. Yan, L. Tian, M. He, and X. Chen, Nano Lett. 15, 6015 (2015).
M.A. Sayeed, T. Herd, and A.P. O’Mullane, J. Mater. Chem. A 4, 991–999 (2016).
M.S. Faber, M.A. Lukowski, Q. Ding, N.S. Kaiser, and S. Jin, J. Phys. Chem. C 118, 21347 (2014).
C. Tang, N. Cheng, Z. Pu, W. Xing, and X. Sun, Angew. Chem. Int. Ed. 54, 9351 (2015).
X. Li, L. Zhang, M. Huang, S. Wang, X. Li, and H. Zhu, J. Mater. Chem. A 4, 14789 (2016).
Z. Fang, L. Peng, H. Lv, Y. Zhu, C. Yan, S. Wang, P. Kalyani, X. Wu, and G. Yu, ACS Nano 11, 9550 (2017).
H. Wang, Y. Li, R. Wang, B. He, and Y. Gong, Electrochem. Acta 284, 504 (2018).
Y. Li, H. Wang, Y. Li, Q. Wang, D. Li, R. Wang, B. He, and Y. Gong, J. Catal. 364, 48 (2018).
C. Guan, X. Liu, W. Ren, X. Li, C. Cheng, and J. Wang, Adv. Energy Mater. 7, 1602391 (2017).
S. Chen, G. Ma, Q. Wang, S. Sun, T. Hisatomi, T. Higashi, Z. Wang, M. Nakabayashi, N. Shibata, Z. Pan, T. Hayashi, T. Minegishi, T. Takata, and K. Domen, J. Mater. Chem. A 7, 7415 (2019).
S. Sahoo, P. Pazhamalai, K. Krishnamoorthy, and S.J. Kim, Electrochem. Acta 268, 403 (2018).
Y. Zhu, Z. Huang, Z. Hu, L. Xi, X. Ji, and Y. Liu, Electrochem. Acta 269, 30 (2018).
Y. Tian, Y. Ruan, J. Zhang, Z. Yang, J. Jiang, and C. Wang, Electrochem. Acta 250, 327 (2017).
X. Hou, P. Xie, S. Xue, H. Feng, L. Li, Z. Liu, and R. Zou, Mater. Sci. Semicond. Process. 79, 92 (2018).
L. Li, F. Zhang, Y. Ding, Y. Wang, and L. Zhang, J. Fluoresc. 19, 437 (2009).
S. Shit, S. Chhetri, W. Jang, N.C. Murmu, H. Koo, P. Samanta, T. Kuila, and A.C.S. Appl, Mater. Interfaces 10, 27712 (2018).
M.M. Peng, U.J. Jeon, M. Ganesh, A. Aziz, R. Vinod, M. Palanichamy, and H.T. Jang, Bull. Korean Chem. Soc. 35, 3213 (2014).
H.H. Zhao, H.L. Song, and L. Chou, J. Inorg. Chem. Commun. 15, 261 (2012).
S. Bordiga, C. Lamberti, G. Ricchiardi, L. Regli, F. Bonino, A. Damin, K.P. Lillerud, M. Bjorgen, and A. Zecchina, Chem. Commun. 20, 2300 (2004).
F. Qiao, L. Chen, X. Li, L. Li, and S. Ai, Sens. Actuators B 193, 255–262 (2014).
F. Qiao, Z. Wang, K. Xu, and S. Ai, Analyst 140, 6684 (2015).
S.K. Warkhade, S.P. Zodape, U.R. Pratap, and A.V. Wankhade, J. Mol. Liq. 279, 434 (2019).
M.S. Begum and A.J. Ahamed, J. Chem. Pharm. Res. 7, 2031 (2015).
P. Shyam, S. Chaturvedi, K. Karmaker, A. Bhattacharya, S. Singh, and S. Kulkarni, J. Mater. Chem. C 4, 611 (2016).
A.J. Ahamed, K. Ramar, and P.V. Kumar, J. Nanosci. Tech. 2, 148 (2016).
Y.H. Hu and L. Zhang, Phys. Rev. B 81, 174103 (2010).
J. Xing, K. Guo, Z. Zou, M. Cai, J. Du, and C. Xu, Chem. Commun. 54, 7046 (2018).
G. Mei, H. Liang, B. Wei, H. Shi, F. Ming, X. Xu, and Z. Wang, Electrochem. Acta 290, 82 (2018).
M. Fiaz and M. Athar, ChemistrySelect 4, 8508 (2019).
Acknowledgements
This project is financially supported by the Higher Education Commission (HEC) of Pakistan under the International Research Support Initiative (IRSIP) program. The authors also acknowledge Prof. Duncan H. Gregory, School of Chemistry, University of Glasgow, UK, for facilitating laboratory facilities to carry out this project.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Fiaz, M., Athar, M. Facile Room-Temperature In Situ Incorporation of Transition-Metal Selenide (TMSe) Nanoparticles into MOF-5 for Oxygen Evolution Reaction. JOM 72, 2219–2225 (2020). https://doi.org/10.1007/s11837-019-03867-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11837-019-03867-0