Abstract
Background
Obstructive sleep apnea (OSA) is a common chronic disorder and an independent risk factor for several health issues, with a high prevalence estimated at 30% for men and 13% for women in Germany.
Objective
For both individual wellbeing and healthcare systems, efficient and effective diagnosis and treatment of OSA are essential. Actors and stations along the patient pathway that may strongly benefit from adoption of current and evolving digital methods and tools are to be identified.
Materials and methods
This work analyzes an OSA patient’s pathway through the German healthcare system, as well as current developments in health informatics and patient involvement. Potential benefits are identified and a patient-centric integrated digital health system is conceptualized.
Results
Digital health strategies of German and European governments emphasize the importance of connected healthcare for patient empowerment, efficient health systems, and innovations in healthcare. For OSA, in particular intersectoral sharing of health assessments and biosignal measurements can support physicians’ care and timely and adequate treatment. Furthermore, clinical decision-support systems including artificial intelligence may help in optimized patient-centric treatment by early detection of females suffering from OSA, OSA pheno- and endotypes, and patients at risk of abandoning treatments. However, bureaucratic and reimbursement barriers in legislation may slow down or even inhibit the implementation of a smart healthcare system.
Conclusion
Current trends in connected digital healthcare, wearables, data-driven decision support, and patient participation offer many opportunities for significantly improving healthcare for OSA. However, many technical, organizational, and regulatory challenges are to be faced.
Zusammenfassung
Hintergrund
Die obstruktive Schlafapnoe (OSA) ist mit einer geschätzten Prävalenz in Deutschland von 30 % bei Männern und 13 % bei Frauen eine häufige chronische Erkrankung sowie ein unabhängiger Risikofaktor diverser Gesundheitsprobleme.
Ziel der Arbeit
Die effiziente und effektive Versorgung von OSA ist essenziell für den Einzelnen und das Gesundheitssystem. Ziel dieses Artikels ist die Identifikation aktueller digitaler Methoden und Werkzeuge zur Verbesserung die Gesundheitsversorgung bei OSA.
Materialien und Methoden
Die Autoren analysieren den Weg einer Person mit OSA durch das deutsche Gesundheitssystem sowie aktuelle Trends der Gesundheitsinformatik und der Patientenbeteiligung. Auf Basis relevanter Verbesserungsmöglichkeiten wird ein Konzept für eine patientenzentrierte integrierte digitale Infrastruktur entwickelt.
Ergebnisse
Die Digital-Health-Strategien der deutschen und europäischen Regierungen betonen die Bedeutung vernetzter Gesundheitsversorgung für Patient-Empowerment sowie Effizienz und Innovationen im Gesundheitswesen. Bei OSA kann insbesondere der sektorenübergreifende Austausch von Gesundheitsdaten und Biosignalmessungen eine rechtzeitige und angemessene ärztliche Behandlung unterstützen. Klinische Entscheidungsunterstützung, inklusive künstlicher Intelligenz, kann bei der optimierten patientenzentrierten Versorgung durch bessere Erkennung von OSA bei Frauen, von Endo- und Phänotypen und von Personen mit hohem Risiko für Therapieabbruch helfen. Allerdings können bürokratische Hürden und eine fehlende Vergütung die Umsetzung erheblich verlangsamen oder sogar verhindern.
Schlussfolgerung
Aktuelle Trends im Bereich der digitalen Gesundheit und Patientenbeteiligung könnten die Versorgung bei OSA deutlich verbessern. Dafür sind technische, organisatorische und regulatorische Herausforderungen zu bewältigen.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
With its high prevalence and as an independent risk factor for many health issues such as neurocognitive function and cardiovascular diseases, obstructive sleep apnea (OSA) is a relevant target to improve individual and public health [30]. In Germany alone, more than 26 million patients demonstrate the need for optimized OSA care. Current trends in digital health are promising for supporting the mission to provide this—from intersectoral data sharing along the patient pathway, to clinical decision support based on artificial intelligence (AI) and wearable devices. Patient participation is crucial in this process.
To employ these new methods for better care in OSA, the interdisciplinary Somnolink consortium encompassing experts in sleep medicine and medical informatics from six university medical centers in Germany was formed in 2022. In the present article, the authors describe current trends in both disciplines and conceptualize how these can be brought together.
Trends in obstructive sleep apnea
OSA is a common disorder that can present with or without symptoms and is accompanied by major neurocognitive and cardiovascular sequelae [24, 30]. The prevalence increases with comorbid cardiovascular diseases, body mass index, and age [21]. The prevalence of moderate to severe OSA in the German population was investigated in the SHIP-TREND study and estimated to be 30% for men and 13% for women [20]. This indicates that almost 14 million people have moderate to severe OSA, for which treatment is generally recommended [29, 30].
Obstructive sleep apnea in women
In contrast to the epidemiological data, only 20% of OSA patients presenting in a German sleep lab are female [25, 29]. This might be due to the fact that symptoms considered typical for OSA, in particular loud and irregular snoring, witnessed apneas, and excessive daytime sleepiness, are less often reported in women [25]. A recent review reported insomnia, depression or mood disturbances, daytime fatigue, and morning headache as clinical symptoms in females [18]. These symptoms largely overlap with symptoms for other sleep disorders, such as insomnia and restless legs syndrome, and psychiatric disorders. Therefore, OSA in women is frequently diagnosed during a stay in the sleep lab initiated by another tentative sleep-related diagnosis. With/after menopause, the risk of clinically relevant OSA becomes evident, resulting in a decreasing gender difference with increasing age [22]. A recent editorial underscores the urgent need for scientific evidence regarding OSA in women [19].
Obstructive sleep apnea in insomnia and depression
In Germany, OSA was found by polysomnography (PSG) in 22.6% of patients with insomnia without any former anamnestic evidence [27]. Psychiatric disorders also increase the risk of OSA. In Germany, 20–25% patients with clinically relevant depression also suffer from OSA [28]. Randomized controlled trials show that treatment of OSA can reduce depressive symptoms.
Obstructive sleep apnea in single households
As complaints by a bed partner are considered relevant hints for suspecting OSA [2], persons without long-term bed partners may be systematically underdiagnosed and symptoms such as loud and irregular snoring might first be noticed when the person shares a room, e.g., during a hospital stay.
Diagnostic and treatment pathways in Germany
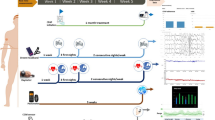
In Germany, a statutory guideline on the diagnosis and treatment of sleep apnea is defined within the Richtlinie Methoden vertragsärztliche Versorgung. The latter has been developed since 1991 by the Joint Federal Committee to define the outpatient medical services that can be reimbursed [14] and defines for OSA a four-step strategy. Step one (medical history) and step two (clinical examinations) are carried out by a general practitioner (GP) to assess the so-called pretest probability—the probability that the patient’s complaints are caused by unrecognized OSA. Unattended home sleep apnea testing (in Germany typically referred to as a polygraphy, PG) has been introduced as step three in the default OSA diagnosis procedure. It is to be carried out by specifically trained and certified outpatient specialists. Comprehensive sleep lab diagnostics with cardiorespiratory PSG for up to two subsequent nights can be carried out as step four, if PG is not sufficient for diagnosis or a treatment decision. Standard OSA treatment is nocturnal positive airway pressure therapy (PAP). PAP is delivered via a mask, which is intended to keep the airways open during sleep and represents an effective treatment for OSA. Adjustment of the PAP pressure, the so-called titration, is to be realized in up to two subsequent nights (treatment initiation) in a sleep lab. The treatment pathway for PAP is well defined, including treatment monitoring, control, and adjustment. Figure 1 illustrates the different actors and relevant sleep data in the OSA patient’s journey. It can be seen from the figure that various types of health data are generated by different stakeholders within the health system and that these are needed in the subsequent steps of diagnosis and treatment of OSA. In contrast to other domains, the long-term measurement of physiological signals—from breathing and heart frequency to multidimensional overnight recordings—is an inherent part of the clinical routine. However, it currently lacks support for patients not tolerating or not experiencing symptom relief from PAP therapy. Modifications of the German treatment pathway are needed to identify such patients and provide options to switch to or other treatment modalities to use primarily for OSA according to the German S3 guideline, such as structured weight loss programs, bariatric surgery, mandibular advancement devices, hypoglossus nerve stimulation, posture therapy, upper airway surgery, or pharmacological therapy [29].
Trends in digital health
The potential benefits of a well-connected digital healthcare system, clinical decision-support systems, and integration of personal device monitoring into the treatment pathway have long since been stated (e.g., [1]). However, implementation of such a system varies strongly between different countries [15].
Connected health
In Germany, we face a strong separation between the healthcare sectors and their distinct stakeholders. The sectors have completely different reimbursement schemes that are even more diverse due to the federal structure of Germany. This has led to a competitive rather than a collaborative relationship between the different sectors, resulting in a highly fragmented system. The information loss at the transition between the different sectors and even between healthcare providers within the same sector impedes optimal care quality and increases costs [7]. Attempts by national legislation in the past two decades have reduced regulatory burden, provided incentives, and resulted in a fund to investigate new forms of care. But despite numerous healthcare reforms, cross-sectoral care has only slightly improved: digitalization with process optimization may shift workload and responsibilities between healthcare actors, which implies renegotiation of roles and duties. Different state data protection laws make it difficult to achieve solutions that are accepted nationwide [12]. Health information technology in Germany is largely provided by small and medium enterprises that claim difficulties with timely implementation of mandatory interoperability standards, and respective deadlines are typically postponed. However, the near monopolistic provision of the so-called telematic infrastructure, Germany’s national secure network for health information exchange, by, ultimately, two companies, has also been criticized for its high costs and negative impacts on functionality. On the other hand, digital health apps can be prescribed and are reimbursed by statutory health insurances in Germany, provided they are certified and enlisted by the Federal Institute for Drugs and Medical Devices. The recently published “Digitalization strategy for the health system and nursing” of the German Ministry of Health envisions 80% of all communication in medicine and nursing to be paperless in 2026, with establishment of digitally supported intersectoral and interprofessional care processes, and will enforce uptake of the electronic health record with an opt-out for citizens [11]. Moreover, the European Commission announced for the European Health Data Space in 2022 [9], with key aspects for primary use of health data, among other things, that “Patients will have their electronic health data available via access points established by Member States […] will be empowered to control and share their electronic health data with a healthcare provider of their choice” and “To support data being shared between healthcare providers, mandatory requirements for interoperability, security, safety and privacy will be introduced.”
Patient participation
A notable shift towards the empowerment of citizens and patients is observed in current governmental statements on digital health, acknowledging the sensitive role of health data for self-determination and privacy. Handling of patient data by medical doctors is strongly safeguarded through medical confidentiality within the patient–doctor relationship, with few exceptions defined by specific laws, such as those related to remuneration, cancer registries, or infection protection. Therefore, the only actors who have the right to decide on sharing health data beyond urgent medical need are the citizens themselves. But patient participation is today regarded as more than just signing informed consent. Active engagement may be implemented along the continuum of consultation, involvement, partnership, and shared leadership on the levels from direct care, organizational redesign, and evaluation of healthcare delivery, up to policy making [16].
Data protection and security
True patient ownership of health data can only be achieved if a seamless, secure, and privacy-preserving option exists for sharing health data along the patient pathway. Advanced cryptographic methods available today can provide robust protection of data at rest and in transit, while providing authorized access [6].
Wearable devices and clinical decision-support systems
Wearable health trackers such as smart watches have gained widespread popularity in the lifestyle sector, and integration of such devices into the regular health system is typically acknowledged as beneficial. They can provide health information over extended periods in daily environments, complementary to clinical assessments [26]. However, the new Medical Device Regulation (MDR) of the European Union (EU) states, “Software intended to monitor physiological processes is classified as class IIa” and the same holds for “Software intended to provide information which is used to take decisions with diagnosis or therapeutic purposes” [8]. Only software “which solely record, store or display information would generally not be considered devices”; all other software is regarded as class I. All medical devices (MDs) must be certified before used in healthcare, i.e., receive a certificate of conformance from an appointed authority. For example, an app that records questionnaires and integrates data from a smart watch is considered an MD. Smart clinical decision-support systems that employ AI are not only considered an MD, but also fall under the currently proposed European AI act, which classifies MDs generally as high-risk systems [13] that “will be assessed before being put on the market and also throughout their life-cycle.” To date, the act is not yet passed, but the bureaucratic burden is expected to be similar to MDR certification. Therefore, implementation of telemedical systems where the attending physician has access to wearable data and can employ AI-based analysis methods might be difficult to realize if the additional costs for development and market entry are not expected to be compensated [4].
Chances and challenges of digital support in obstructive sleep apnea care
Sleep data sharing
Main information sources during OSA diagnosis are texts from questionnaires, clinical documentation, and diagnostic reports. Here, interoperable digital data, i.e., readable and correctly interpretable by different health information systems, would allow sharing and visualization of relevant information for all actors along the patient pathway. While digital questionnaires are common in healthcare, interoperability and the licensing of digital versions are still challenging and have hindered broad availability of digital sleep assessments beyond local solutions. Clinical documentation such as doctors’ letters are, to date, typically nonstructured text files, although a German standard for structured doctors’ letters has existed since 2014. While machine learning methods, e.g., natural language processing models and large language models such as ChatGPT (OpenAI; San Francisco, CA, USA), are capable of condensing unstructured text into comprehensible structured information, the output quality may strongly rely on the input quality, e.g., the completeness of the information. As such methods would be considered high risk according to the EU AI act, the balance between effort and benefit might be not sufficient for implementation. A more expedient solution might be documentation of a limited consented number of structured data alongside the unstructured text. Diagnostic reports are also provided typically as text documents, although they are typically structured or semistructured, reporting predefined parameters. In this context, a promising option is to agree on standardized exchange formats and common terminologies with the medical device manufacturers that create and pre-fill the reports.
In OSA, overnight biosignal recordings are highly relevant. PGs and PSGs record at least breathing and heart rate, but up to 40 different physiological measures are possible, and PAP devices record usage statistics, mask pressure, and breathing behavior. While manufacturers offer local or cloud-based data management, visualization, and report generation from the biosignal recordings, no interinstitutional data-sharing infrastructure has yet been established for primary care. Currently, a nationally funded project is attempting to merge diagnostic data generated by the various actors in the patient pathway on a collaborative project platform. However, these only represent aggregated final diagnostic data to bridge the intra- and intersectoral information gaps [10]. Compared to medical imaging where the Digital imaging and Communication in Medicine (DICOM) standard is used, data and meta-data standards are not as widely supported by biosignal devices. The European Data Format is established as a scientific exchange format, but leaves options in a nonharmonized manner and provides a very limited set of meta-data [5]. A DICOM standard for PSG has recently been defined and awaits adoption by the vendors [3]. Based on previous work on collaboration in sleep medicine, adoption to share and visualize overnight biosignal recordings and extend to wearable and therapeutic devices data is a promising solution. A preliminary architecture for such an infrastructure has been developed by the Somnolink consortium and is shown in Fig. 2. Question marks show possible data flow directions that must be considered carefully and in particular with involvement of patients into the design process. Regarding certification under the MDR or under the AI act, any data processing may cause the whole system to become a medical device or high-risk system and must be carefully conceptualized.
Clinical decision support in obstructive sleep apnea care
Several use cases for data-driven decision-support systems are of high interest in OSA care. In sleep medicine, diagnosis and a personalized treatment decision requires consideration of the patient’s personal circumstances as well as all their diseases. In this complex setting, data-driven decision-support systems could synergistically complement physicians’ knowledge and experience, thus improving overall patient care. Detection of possible OSA patients in underdiagnosed groups—such as women or persons living in single households—could be supported by screening of relevant information from hospital stays. This becomes feasible by the current activities of the Medical Informatics Initiative that integrates and harmonizes clinical information in the so-called medical data integration centers in all German university medical centers [23]. The identification of OSA pheno- and endotypes that respond to specific treatment methods may have a major impact on OSA healthcare in the future. The identification of persons who feel uncomfortable with the current treatment and are at high risk of abandoning it is of equal importance [17]. Clinical assessment, monitoring, and patient-reported outcome measures are assumed to play an important role in both scenarios. Again, to enable transfer of innovative methods into practice, careful design of the development and validation process is required.
Conclusion
Trends in digital health are promising for better OSA care through implementation of improved information sharing and integration of clinical decision support based on wearable devices and patient-reported outcomes into the care process. This can be only achieved if all actors are positive about the benefits, trust the safety and security measures, and actively engage in implementation of a connected digital healthcare system. Finally, implementation into clinical routine will require feasible health care regulations as well as reimbursement. The authors consider the multidisciplinary Somnolink consortium to be a promising starting point for adopting innovative methods for better OSA healthcare in Germany. In 2024, the consortium will commence as part of the Medical Informatics Initiative to investigate and implement the aforementioned approaches to improving OSA care by leveraging the potentials of digitalization. As many regulatory aspects are within the framework of the European Union and many methods are also relevant for other sleep-related disorders, we envision an impact beyond country and medical domains.
References
6th IEEE International Conference on Digital Ecosystems and Technologies (DEST 2012). https://doi.org/10.1109/DEST.2012.6227929 (Personal health systems for diagnostics of sleep disorders using new sensors and grid technology)
Chest (2014). https://doi.org/10.1378/chest.13-1046 (The utility of the elbow sign in the diagnosis of OSA)
Clin Neurophysiol (2021). https://doi.org/10.1016/j.clinph.2021.01.019 (Standardization of neurophysiology signal data into the DICOM® standard)
Frontiers in Public Health (2021) https://doi.org/10.3389/fpubh.2021.666453 (Do Regulatory Changes Seriously Affect the Medical Devices Industry? Evidence From the Czech Republic)
Future Generation Computer Systems (2017). https://doi.org/10.1016/j.future.2016.03.025 (Multicenter data sharing for collaboration in sleep medicine)
GMS (2020) https://doi.org/10.3205/20gmds163 (ASCLEPIOS: Sharing and Analysing Healthcare Data in the Cloud with New Cryptographic Methods)
Germany: health system summary | European Observatory on Health Systems and Policies. https://eurohealthobservatory.who.int/publications/i/germany-health-system-summary-2022. Accessed 27 June 2023
MDCG 2021-24—Guidance on classification of medical devices. https://health.ec.europa.eu/latest-updates/mdcg-2021-24-guidance-classification-medical-devices-2021-10-04_en. Accessed 27 June 2023
Communication from the Commission—A European Health Data Space: harnessing the power of health data for people, patients and innovation. https://health.ec.europa.eu/publications/communication-commission-european-health-data-space-harnessing-power-health-data-people-patients-and_en. Accessed 25 June 2023
SLEEP WELL – Digital unterstützte Schlafmedizin – Entwicklung optimierter Patientenwege bei obstruktiver Schlafapnoe – G‑BA Innovationsfonds. https://innovationsfonds.g-ba.de/projekte/neue-versorgungsformen/sleep-well-digital-unterstuetzte-schlafmedizin-entwicklung-optimierter-patientenwege-bei-obstruktiver-schlafapnoe.433. Accessed 27 June 2023
Digitalisierungsstrategie. https://www.bundesgesundheitsministerium.de/themen/digitalisierung/digitalisierungsstrategie.html. Accessed 25 June 2023
Gesetze und Verordnungen – Virtuelles Datenschutzbüro. https://www.datenschutz.de/home/gesetze-und-verordnungen/. Accessed 27 June 2023
EU AI Act: first regulation on artificial intelligence | News | European Parliament. https://www.europarl.europa.eu/news/en/headlines/society/20230601STO93804/eu-ai-act-first-regulation-on-artificial-intelligence. Accessed 25 June 2023
Richtlinie Methoden vertragsärztliche Versorgung – Gemeinsamer Bundesausschuss. https://www.g-ba.de/richtlinien/7/. Accessed 27 June 2023
Health at a Glance: Europe 2022 : State of Health in the EU Cycle | Health at a Glance: Europe | OECD iLibrary. https://www.oecd-ilibrary.org/social-issues-migration-health/health-at-a-glance-europe-2022_507433b0-en. Accessed 27 June 2023
Implement Sci (2018). https://doi.org/10.1186/s13012-018-0784-z (Engaging patients to improve quality of care: a systematic review)
J Clin Med (2019). https://doi.org/10.3390/jcm8122220 (Novel Aspects of CPAP Treatment and Interventions to Improve CPAP Adherence)
J Clin Sleep Med (2019). https://doi.org/10.5664/jcsm.8074 (Cardiovascular Effect and Symptom Profile of Obstructive Sleep Apnea: Does Sex Matter?)
J Clin Sleep Med (2022) . https://doi.org/10.5664/jcsm.9684 (Obstructive sleep apnea in women: scientific evidence is urgently needed)
J Sleep Res (2019). https://doi.org/10.1111/jsr.12770 (Prevalence and association analysis of obstructive sleep apnea with gender and age differences—Results of SHIP-Trend)
JACC Heart Fail (2016). https://doi.org/10.1016/j.jchf.2015.09.014 (Prevalence and Predictors of Sleep-Disordered Breathing in Patients With Stable Chronic Heart Failure: The SchlaHF Registry)
Maturitas (2019). https://doi.org/10.1016/j.maturitas.2019.02.011 (Menopause and Sleep Apnea)
Methods Inf Med (2018). https://doi.org/10.3414/ME18-03-0003 (German Medical Informatics Initiative)
Nat Rev Dis Primers (2015). https://doi.org/10.1038/nrdp.2015.15 (Obstructive sleep apnoea syndrome)
Pneumologie (2007). https://doi.org/10.1055/s-2007-980128 ([Sleep apnoea in women?—The forgotten gender])
Sleep (2022). https://doi.org/10.1093/sleep/zsac183 (Sleep characterization with smart wearable devices: a call for standardization and consensus recommendations)
Sleep Breath (2012). https://doi.org/10.1007/s11325-011-0608-8 (Polysomnography reveals unexpectedly high rates of organic sleep disorders in patients with prediagnosed primary insomnia)
Sleep Breath (2017). https://doi.org/10.1007/s11325-016-1411-3 (Obstructive sleep apnea (OSA) and clinical depression-prevalence in a sleep center)
Somnologie (2022). https://doi.org/10.1007/s11818-022-00349-5 (Partial update of the German S3 Guideline Sleep-Related Breathing Disorders in Adults)
The Lancet Respiratory Medicine (2019). https://doi.org/10.1016/S2213-2600(19)30198-5 (Estimation of the global prevalence and burden of obstructive sleep apnoea: a literature-based analysis)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
M. Arzt has received consulting fees from ResMed and Philips Respironics, and grant support from the ResMed Foundation, Philips Respironics, and the Else-Kroener Fresenius Foundation (2018_A159). J.T. Maurer has received grants, consulting, and/or honorary fees from Nyxoah, LivaNova, Inspire, XM Consulting, Neuwirth, and Medel. T. Penzel has received consulting and honorary fees from Löwenstein Medical, Bayer, Jazz Pharma, Bioprojet, Idorsia, Cerebra, Philips, Neuwirth, and the National Sleep Foundation, grant support from Cidelec and Sleepimage, and participates in the companies The Siestagroup und Advanced Sleep Research. D. Krefting, F. Prasser, M. Sedlmayr, and C. Schöbel declare that they have no competing interests.
For this article no studies with human participants or animals were performed by any of the authors. All studies mentioned were in accordance with the ethical standards indicated in each case.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.

Scan QR code & read article online
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Krefting, D., Arzt, M., Maurer, J.T. et al. Sleep apnea healthcare management in dynamically changing times. Somnologie 27, 248–254 (2023). https://doi.org/10.1007/s11818-023-00428-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11818-023-00428-1
Keywords
- Obstructive sleep apnea
- Digital technology
- Intersectoral collaboration
- Wearable electronic devices
- Patient participation